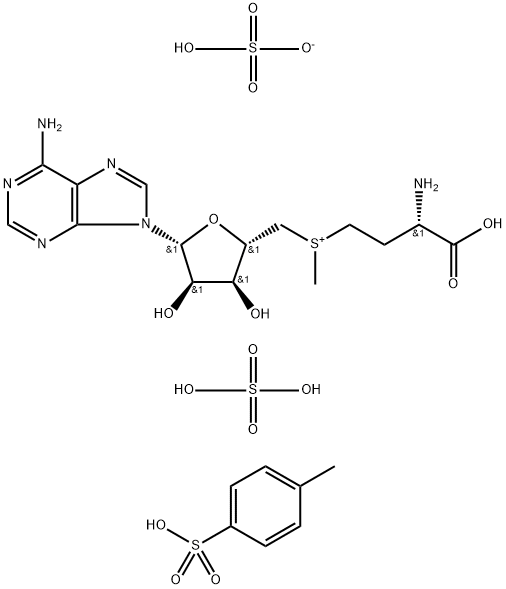
5'-DEOXY-5'-METHYLTHIOADENOSINE
Synonym(s):5ʹ-Deoxy-5ʹ-methylthioadenosine - CAS 2457-80-9 - Calbiochem;Methylthioadenosine;MTA;MTA, MeSAdo
- CAS NO.:2457-80-9
- Empirical Formula: C11H15N5O3S
- Molecular Weight: 297.33
- MDL number: MFCD00010533
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
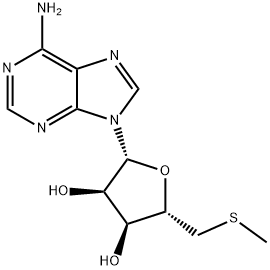
What is 5'-DEOXY-5'-METHYLTHIOADENOSINE?
The Uses of 5'-DEOXY-5'-METHYLTHIOADENOSINE
5′-Deoxy-5′-(methylthio)adenosine has been used as a protein methylation inhibitor to reduce E2F transcription factor 1 (E2F1) protein abundance in hepatocellular carcinoma (HCC) cells. It has also been used to inhibit histone methylation modification and study its role in hypoxia inducible factor-1 (Hif-1) nuclear transport.
What are the applications of Application
5′-Deoxy-5′-methylthioadenosine is an endogenous methylthioadenosine phosphorylase substrate and potent inhibitor of PDE
Definition
ChEBI: Adenosine with the hydroxy group at C-5' substituted with a methylthio (methylsulfanyl) group.
Biological Activity
ki = 62 μm for s49-derived high-affinity camp phosphodiesterasemethylthioadenosine (mta) is a naturally occurring sulfur-containing nucleoside in all mammalian tissues. mta is mainly produced from s-adenosylmethionine via the polyamine biosynthetic pathway, behaving as a powerful inhibitory product.
Biochem/physiol Actions
5′-Deoxy-5′-(methylthio)adenosine (Methylthioadenosine) may be used as a substrate to study the specificity and kinetics of 5′-methylthioadenosinephosphorylase (MTAP) (EC2.4.2.28), a tumor suppressor gene expressed enzyme that supports the S-adenosylmethionine (AdoMet) and methionine salvage pathways.
in vitro
mta was found to be solely metabolized by mta-phosphorylase, to yield 5-methylthioribose-1-phosphate and adenine, which was a key step in methionine and purine salvage pathways, respectively. previous studies suggested that mta coud affect various cellular processes. mta had been shown to be albe to influence plenty of critical cell responses, such as proliferation, gene expression regulation, differentiation as well as apoptosis. though most of such responses had been only observed at the pharmacological level, their specificity made it tempting to speculate that endogenous mta might play a regulatory role in the cell [1].
in vivo
the hepatoprotective effects of mta had been evaluated in a model of ccl4-induced chronic liver damage in rats. in this study, mta could reproduce the human lesions induced by alcohol and viral infections of the liver. in addition, replenishment of the hepatic pool of mta showed strong anti-oxidant effects and also reduced liver cell damage and fibrosis [2].
Purification Methods
Recrystallise it from H2O and sublime it at 200o/0.004mm. [v.Euler & Myrb.ck Hoppe Seyler's Z physiol Chem 177 237 1928, Weygand & Trauth Chem Ber 84 633 1951, Baddiley et al. J Chem Soc 2662 1953.] The hydrochloride has m 161-162o [Kuhn & Henkel Hoppe Seyler's Z Physiol Chem 269 41 1941]. The picrate has m 183o(dec) (from H2O). [Beilstein 26 III/IV 3675.]
References
[1] avila ma,garcía-trevijano er,lu sc,corrales fj,mato jm. methylthioadenosine. int j biochem cell biol.2004 nov;36(11):2125-30.
[2] pascale rm,simile mm,de miglio mr,feo f. chemoprevention of hepatocarcinogenesis: s-adenosyl-l-methionine. alcohol.2002 jul;27(3):193-8.
Properties of 5'-DEOXY-5'-METHYLTHIOADENOSINE
| Melting point: | 210-213 °C (dec.) |
| Boiling point: | 642.7±65.0 °C(Predicted) |
| Density | 1.85±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMF (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | White solid |
| pka | 13.10±0.70(Predicted) |
| color | White to Off-White |
| BRN | 42420 |
Safety information for 5'-DEOXY-5'-METHYLTHIOADENOSINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 5'-DEOXY-5'-METHYLTHIOADENOSINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![ADENOSYL-L-METHIONINE, S-[CARBOXYL-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB7362189.gif)


![S-ADENOSYL-L-[METHYL-3H]METHIONINE](https://img.chemicalbook.in/CAS/GIF/111093-45-9.gif)
You may like
-
 5′-Deoxy-5′-methylthioadenosine CAS 2457-80-9View Details
5′-Deoxy-5′-methylthioadenosine CAS 2457-80-9View Details
2457-80-9 -
 5′-Deoxy-5′-(methylthio)adenosine CAS 2457-80-9View Details
5′-Deoxy-5′-(methylthio)adenosine CAS 2457-80-9View Details
2457-80-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8