CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 156-158 °C |
| Solubility | 18.6 [ug/mL] (The mean of the results at pH 7.4) |
| LogP | -1.5 |
| Collision Cross Section | 130.7 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with ESI Low Concentration Tuning Mix (Agilent)] |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 131.13 g/mol |
|---|---|
| XLogP3 | -3.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 131.058243149 g/mol |
| Monoisotopic Mass | 131.058243149 g/mol |
| Topological Polar Surface Area | 80.4 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
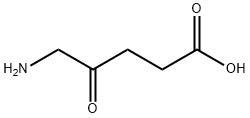
5-aminolevulinic acid is the simplest delta-amino acid in which the hydrogens at the gamma position are replaced by an oxo group. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used (in the form of the hydrochloride salt)in combination with blue light illumination for the treatment of minimally to moderately thick actinic keratosis of the face or scalp. It has a role as a photosensitizing agent, an antineoplastic agent, a dermatologic drug, a prodrug, a plant metabolite, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a delta-amino acid and a 4-oxo monocarboxylic acid. It is functionally related to a 4-oxopentanoic acid. It is a conjugate base of a 5-ammoniolevulinic acid. It is a conjugate acid of a 5-aminolevulinate. It is a tautomer of a 5-ammoniolevulinate.