4-Vinyl-1-cyclohexene
Synonym(s):4-Ethenyl-1-cyclohexene;NSC 15760
- CAS NO.:100-40-3
- Empirical Formula: C8H12
- Molecular Weight: 108.18
- MDL number: MFCD00001576
- EINECS: 202-848-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-06-13 14:48:16
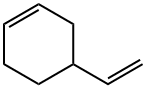
What is 4-Vinyl-1-cyclohexene?
Chemical properties
Liquid. Temperatures above 80F (26.6C) and prolonged exposure to oxygen-containing gases should be avoided because these conditions lead to discoloration and gum formation.
Chemical properties
4-Vinyl-1-cyclohexene, a cyclic alkene, is a highly flammable liquid.
The Uses of 4-Vinyl-1-cyclohexene
Polymers, organic synthesis.
The Uses of 4-Vinyl-1-cyclohexene
As an intermediate in the production of flame retardants, flavors, fragrances, and vinyl cyclohexene dioxide (which itself is used in the manufacture of epoxy resins); found in gases discharged during the process of curing rubber in tire manufacturing.
Production Methods
4-Vinylcyclohexene is produced by dimerization of butadiene, as a by-product of chlorination of butadiene, or frombutadiene on long storage.
Definition
ChEBI: 4-Vinylcyclohexene is a cyclic olefin.
Synthesis Reference(s)
Journal of the American Chemical Society, 108, p. 1322, 1986 DOI: 10.1021/ja00266a047
Synthesis, p. 1027, 1984
General Description
Colorless liquid. Floats on water. Temperatures above 80F (26.6C) and prolonged exposure to oxygen-containing gases should be avoided because these conditions lead to discoloration and gum formation.
Air & Water Reactions
Highly flammable. 4-Vinyl-1-cyclohexene oxidizes in air to form hydroperoxides. Slightly soluble in water.
Reactivity Profile
4-Vinyl-1-cyclohexene can react with oxidizing materials. 4-Vinyl-1-cyclohexene is also incompatible with peroxide catalysts. Prolonged exposure to oxygen-containing gases leads to discoloration and gum formation. .
Health Hazard
Exposure can cause irritation of eyes, nose and throat. High concentrations have a narcotic effect.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Moderately toxic by ingestion and inhalation. MilcU~7 toxic by skin contact. Experimental reproductive effects. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Can react with oxidizers. To fight fire, use foam, CO2, dry chemical.
Potential Exposure
4-Vinyl-1-cyclohexene is used as an intermediate for the production of vinylcyclohexene dioxide, which is used as a reactive diluent in epoxy resins. Previous uses of 4-vinyl-1-cyclohexene include comonomer in the polymerization of other monomers and for halogenation to polyhalogenated derivatives which are used as flame retardants.
Carcinogenicity
A dose of 145 g/kg applied to mouse skin for 54 weeks provided weak evidence of carcinogenicity (109). Administration by gavage of doses of 0, 200, or 400 mg/kg body weight, 5 days per week, to groups of 50 F344/N rats for 103 weeks induced a slightly increased incidence of epithelial hyperplasia of the forestomach (1/50; 3/50; 5/47) and squamous cell papillomas or carcinomas (combined) of the skin, in males receiving the highest dose. Low-dose female rats whose survival was more similar to that of the vehicle controls had a marginally increased incidence of adenomas or squamous cell carcinomas (combined) of the clitoral gland. Under these conditions, the 2-year gavage studies in male and female rats were considered inadequate because of extensive and early mortality at the high dose or at body doses and the lack of conclusive evidence of a carcinogenic effect (125a). In female Fisher 344 rats treated with this agent to induce ovarian failure followed by application of the carcinogen dimethylbenzanthracene (DMBA) directly to the ovaries, there resulted tumors in 42% of animals at 3 months and 57% at 5 months (126b). All neoplasms were classified Sertoli–Leydig cell tumors (SLCT). No tumors occurred in animals treated with vehicle or DMBA alone.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
Incompatibilities
Vapor may form explosive mixture with air. Hydrolyzes in water. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, amines, alcohols.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of 4-Vinyl-1-cyclohexene
| Melting point: | -101 °C |
| Boiling point: | 126-127 °C(lit.) |
| Density | 0.832 g/mL at 25 °C(lit.) |
| vapor density | 3.76 (vs air) |
| vapor pressure | 10.2 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 68 °F |
| storage temp. | 2-8°C |
| form | Powder |
| color | Off-white to beige-brownish |
| Specific Gravity | 0.832 |
| Water Solubility | 50mg/L(25 ºC) |
| BRN | 1901553 |
| CAS DataBase Reference | 100-40-3(CAS DataBase Reference) |
| IARC | 2B (Vol. Sup 7, 60) 1994 |
| EPA Substance Registry System | 4-Vinylcyclohexene (100-40-3) |
Safety information for 4-Vinyl-1-cyclohexene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H351:Carcinogenicity H361:Reproductive toxicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 4-Vinyl-1-cyclohexene
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![TETRAMETHYL 2,6-DIHYDROXYBICYCLO[3.3.1]NONA-2,6-DIENE-1,3,5,7-TETRACARBOXYLATE](https://img.chemicalbook.in/CAS/GIF/6966-22-9.gif)
![4,4,10-TRIMETHYL TRICYCLO[7,3,1,0(1-6)]-6,10-TRIDECADIENE-2,8-DIONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB5153505.gif)

You may like
-
 4-Vinyl-1-cyclohexane, 99% CAS 100-40-3View Details
4-Vinyl-1-cyclohexane, 99% CAS 100-40-3View Details
100-40-3 -
 4-Vinyl-1-cyclohexene, 99% CAS 100-40-3View Details
4-Vinyl-1-cyclohexene, 99% CAS 100-40-3View Details
100-40-3 -
 4-Vinyl-1-cyclohexene (stabilized with BHT) CAS 100-40-3View Details
4-Vinyl-1-cyclohexene (stabilized with BHT) CAS 100-40-3View Details
100-40-3 -
 4-Vinyl-1-cyclohexene CAS 100-40-3View Details
4-Vinyl-1-cyclohexene CAS 100-40-3View Details
100-40-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
