4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
- CAS NO.:84392-17-6
- Empirical Formula: C14H9F3O2
- Molecular Weight: 266.22
- MDL number: MFCD00075353
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:15
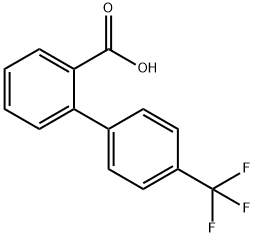
What is 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid?
Chemical properties
White solid
Originator
Xenalipin ,Wellcome (GSK)
The Uses of 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
Hypolipidemic.
The Uses of 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
4''-(Trifluoromethyl)-2-biphenylcarboxylic Acid, is a novel compound which has been found to be effective hypolipidemic agent in animal species. It is also an intermediate in the synthesis of various pharmaceutical compounds, such as Cannabinol (C175350).
Manufacturing Process
a). Preparation of 2-(4,4-dimethyl-2-oxazolin-2-yl)-4'-trifluoromethylbiphenyl:
A mechanically stirred solution of magnesium turnings (5.1 g, 0.21 mole)
Mallinckrodt, for Grignards reaction, and 2-(2-methoxyphenyl)-4,4-dimethyl-
2-oxazoline (41 g, 0.2 mole) in 50 mL dry tetrahydrofuran under nitrogen was
prepared. To this was added a crystal of iodine, 1 mL of dibromethane and 2
mL of neat p-bromotrifluoromethylbenzene to initiate the Grignard reaction.
Following initiation of the reaction, the remainder of the p-
bromotrifluoromethylbenzene (50 g, 0.22 mole total) in 100mL dry
tetrahydrofuran was added dropwise at a rate sufficient to maintain the
reaction at gentle reflux. The addition took 1 hour. At the end of the addition
period, the reaction mixture was heated to reflux for 3 hours. The reaction
mixture was then cooled to room temperature and 10 mL water was added
dropwise to coagulate the salts. The tetrahydrofuran was decanted and the
remaining solids were slurried twice with 300 mL ethyl ether and twice with
300 mL dichloromethane. Each organic extract was decanted from the solids
in turn and combined and evaporated under reduced pressure to an oil.
This oil was redissolved in 300 mL dichloromethane, washed once with 100
mL water and once with 10 mL saturated sodium chloride solution, dried and
concentrated under reduced pressure. The resulting residue was distilled
under reduced pressure (0.040 mm, 95.degree.) to yield 2-(4,4-dimethyl-2-
oxazoline-2-yl)-4'-trifluoromethylbiphenyl, yield 33%. A sample was
recrystallized from 30-60°C petroleum ether (melting point 50-51°C).
b). Preparation of 4'-(trifluoromethyl)-2-biphenylcarboxylic acid:
A solution of 2-(4,4-dimethyl-2-oxazolin-2-yl)-4'-trifluoromethylbiphenyl (3.5
g, 0.011 mole) in 60 mL 6 N hydrochloric acid was stirred at vigorous reflux
for 2 hr. The reaction mixture was then cooled to room temperature and
extracted with methylene chloride. The organic extracts were dried and
concentrated under reduced pressure to yield a solid. Recrystallisation from
ethyl ether/pentane afforded 4'-(trifluoromethyl)-2-biphenyl carboxylic acid,
yield 86% (melting point 167-169°C).
Therapeutic Function
Antihyperlipidemic
General Description
4′-(Trifluoromethyl)-2-biphenylcarboxylic acid (xenalipin) has been tested as an effective hypolipidemic agent in animal species. It has been shown to cause significant reduction in serum cholesterol and triglyceride levels in animal models and would be beneficial in therapy for hyperlipidemia. Synthesis of 4′-(trifluoromethyl)-2-biphenylcarboxylic acid (xenalipin) has been reported. Xenalipin has been synthesized in the [14C]-labelled form, with specific activity 21.0mCi/mmol, which is suitable for the metabolism and distribution studies in animals.
Properties of 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
| Melting point: | 169-171 °C(lit.) |
| Boiling point: | 335.8±37.0 °C(Predicted) |
| Density | 1.326±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | soluble in Methanol |
| form | powder to crystal |
| pka | 3.70±0.36(Predicted) |
| color | White to Almost white |
| BRN | 7577560 |
| CAS DataBase Reference | 84392-17-6(CAS DataBase Reference) |
Safety information for 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 4-(Trifluoromethyl)-2'-biphenylcarboxylic acid
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 4'-(Trifluoromethyl)biphenyl-2-carboxylic Acid CAS 84392-17-6View Details
4'-(Trifluoromethyl)biphenyl-2-carboxylic Acid CAS 84392-17-6View Details
84392-17-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1