4-Nitrobenzyl chloride
Synonym(s):α-Chloro-4-nitrotoluene
- CAS NO.:100-14-1
- Empirical Formula: C7H6ClNO2
- Molecular Weight: 171.58
- MDL number: MFCD00007374
- EINECS: 202-822-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
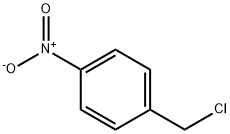
What is 4-Nitrobenzyl chloride?
Description
Benzene, 1-(chloromethyl)-4-nitro- is a crystalline solid. Molecular weight= 171.58; Freezing/Meltingpoint =71℃. Slightly soluble in water; slow reaction.
Chemical properties
white to light yellow crystal powde
Chemical properties
Benzene, 1-(chloromethyl)-4-nitro-, is a crystalline solid.
The Uses of 4-Nitrobenzyl chloride
4-Nitrobenzyl chloride was used to prepare unsymmetrically N,N′-bis(substituted) 4,13-diaza-18-crown-6-ether derivatives.
What are the applications of Application
4-Nitrobenzyl chloride is a GST Substrate
Definition
ChEBI: A C-nitro compound that is nitrobenzene in which the hydrogen at position 4 is replaced by a chloromethyl group.
Production Methods
Chlorination of 4-nitrotoluene at 190 ℃ without catalyst gives 4-Nitrobenzyl chloride. The alternative process of nitration of benzylchloride coproduces 2-nitrobenzyl chloride and requires a difficult separation stage.
General Description
Solid.
Air & Water Reactions
May react slowly with water.
Reactivity Profile
Incompatible with sodium hydroxide. [EPA, 1998]. A halogenated aromatic nitro compound. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
Health Hazard
Poisonous if swallowed or dust is inhaled.
Fire Hazard
Incompatible with sodium hydroxide.
Potential Exposure
A chloronitrobenzene compound, used in organic synthesis.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Store in a refrigerator or in acool, dry place and protect from prolonged exposure tomoisture.
Shipping
UN3261 Corrosive solid, acidic, organic, n.o.s., Hazard class: 8; Labels: 8—Corrosive material, Technical Name Required. UN1578 Chloronitrobenzenes, solid or liquid, Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Purification Methods
Crystallise the chloride from CCl4, dry diethyl ether, or n-heptane, and dry it under vacuum. IRRITANT.[Beilstein 5 IV 856.]
Incompatibilities
Can react with sulfuric acid. Keep away from oxidizers, amines, bases: sodium hydroxide, and potassium hydroxide; may cause fire and explosions. Incompatible with strong oxidizing and reducing agents such as hydrides, certain amines, nitrides, azo/diazo compounds, alkali metals (potassium), and epoxides. Corrodes steel, some plastics and human tissue.
Properties of 4-Nitrobenzyl chloride
| Melting point: | 71 °C |
| Boiling point: | 112 °C (0.6 mmHg) |
| Density | 1.3246 (rough estimate) |
| refractive index | 1.5647 (estimate) |
| solubility | chloroform: soluble50mg/mL, clear to very slightly hazy, faintly yellow |
| form | Crystalline Needles or Powder |
| color | Light yellow |
| BRN | 387187 |
| Stability: | Stable. Incompatible with strong oxidizing agents, bases, amines, moisture, alcohols. Corrodes steel. Reacts slowly with water. Combustible. |
| CAS DataBase Reference | 100-14-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Nitrobenzyl chloride(100-14-1) |
| EPA Substance Registry System | 1-(Chloromethyl)-4-nitrobenzene (100-14-1) |
Safety information for 4-Nitrobenzyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Nitrobenzyl chloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![2-[[(2-ethylphenyl)(2-hydroxyethyl)amino]methyl]-3,3-difluoro-Propanenitrile](https://img.chemicalbook.in/CAS/GIF/2647-14-5.gif)


You may like
-
 4-Nitrobenzyl chloride CAS 100-14-1View Details
4-Nitrobenzyl chloride CAS 100-14-1View Details
100-14-1 -
 4-Nitro benzyl chlorideView Details
4-Nitro benzyl chlorideView Details
100-14-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
