4-Chloronitrobenzene
- CAS NO.:100-00-5
- Empirical Formula: C6H4ClNO2
- Molecular Weight: 157.55
- MDL number: MFCD00007285
- EINECS: 202-809-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
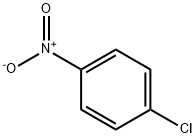
What is 4-Chloronitrobenzene?
Chemical properties
4-Chloronitrobenzene is a light yellow monoclinic prisms crystallizes, which are insoluble in water and very soluble in toluene, ether, acetone, or hot ethanol. It is incompatible with strong oxidizers and alkalis. It is extensively used in different industries as an intermediate in the manufacture of dyes, rubber, and agricultural chemicals.
Physical properties
p-Nitrochlorobenzene is a yellow crystalline solid with a sweet odor.
The Uses of 4-Chloronitrobenzene
4-Nitrochlorobenzene is an important intermediate in the manufacture of azo dyes and sulfide dyes, the drugs finasteride and paracetamol, the pesticide herbicide, etc. It is also the raw material of rubber antioxidant 4010.
The Uses of 4-Chloronitrobenzene
p-Nitrochlorobenzene is largely used to produce p-nitrophenol with smaller production of p-nitroaniline.
The Uses of 4-Chloronitrobenzene
Manufacture of dyes, rubber, and agricultural chemicals
Production Methods
p-Nitrochlorobenzene is made by the nitration of chlorobenzene.
Definition
ChEBI: 4-Chloronitrobenzene is a C-nitro compound.
Synthesis Reference(s)
The Journal of Organic Chemistry, 52, p. 2407, 1987 DOI: 10.1021/jo00388a014
Tetrahedron Letters, 19, p. 4519, 1978
General Description
Light yellow crystalline solid. Density 1.520 g / cm3. Melting point 83°C. Sweet odor. Very toxic by inhalation, ingestion, and skin absorption. p-Chloronitrobenzene is extensively used in different industries as an intermediate in the manufacture of dyes, rubber, and agricultural chemicals. It is incompatible with strong oxidisers and alkalis.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
4-Chloronitrobenzene reacts with oxidizing agents. Reacts violently and finally explosively when added to a solution of sodium methoxide in methanol. . Unstable when heated.
Hazard
A questionable carcinogen. Very toxic by inhalation and ingestion. Absorbed via skin. Combustible. Methemoglobinemia.
Health Hazard
Repeated exposure to high levels of p-chloronitrobenzene causes adverse health effects. The symptoms of toxicity include, but are not limited to, anoxia, unpleasant taste, anemia, methemoglobinemia, hematuria (blood in the urine), spleen, kidney, bone marrow changes, and reproductive effects. The target organs of p-chloronitrobenzene poisoning have been identifi ed as the blood, liver, kidneys, cardiovascular system, spleen, bone marrow, and reproductive system.
Fire Hazard
4-Chloronitrobenzene is combustible.
Safety Profile
Confirmed carcinogen with experimental carcinogenic data. A poison by ingestion. Experimental reproductive effects. Mutation data reported. Flammable liquid when exposed to heat, sparks, or flame. May explode on heating. Potentially violent reaction with sodium methoxide. When heated to decomDosition it emits very toxic fumes of NOx and Cl-. See also other chloronitrobenzene entries and NITRO COMPOUNDS OF AROMATIC HYDROCARBONS.
Potential Exposure
p-Nitrochlorobenzene (PNCB) is used as an intermediate in pesticide (parathion) manufacture, drug (phenacetin and acetaminophen) manufacture; and in dye making; rubber and antioxidant manufacture.
Carcinogenicity
No increase in tumor incidence was seen in rats fed up to 1000 ppm in the diet for 2 years; in mice, results were equivocal, with high-dose animals showing an increase in vascular tumors and low-dose males showing an increase in liver tumors.6 The IARC has determined that there is inadequate evidence in experimental animals and humans for the carcinogenicity of chlorobenzenes.7
Environmental Fate
Biological. Under aerobic conditions, the yeast Rhodosporidium sp. metabolized pchloronitrobenzene
to 4-chloroacetanilide and 4-chloro-2-hydroxyacetanilide as final major
metabolites. Intermediate compounds identified include 4-chloronitrosobenzene, 4-chlorophenylhydroxylamine,
and 4-chloroaniline (Corbett and Corbett, 1981).
Under continuous flow conditions involving feeding, aeration, settling, and reflux, a mixture of
p-chloronitrobenzene and 2,4-dinitrochlorobenzene was reduced 61–70% after 8–13 d by
Arthrobacter simplex, a microorganism isolated from industrial waste. A similar experiment was
conducted using two aeration columns. One column contained A. simplex, the other a mixture of
A. simplex and microorganisms isolated from soil (Streptomyces coelicolor, Fusarium sp.,
probably aquaeductum and Trichoderma viride). After 10 d, 89.5–91% of the nitro compounds
was reduced. p-Chloronitrobenzene was reduced to 4-chloroaniline and six unidentified
compounds (Bielaszczyk et al., 1967).
Photolytic. An aqueous solution containing p-chloronitrobenzene and a titanium dioxide
(catalyst) suspension was irradiated with UV light (λ >290 nm). 2-Chloro-5-nitrophenol was the
only compound identified as a minor degradation product. Continued irradiation caused additional
degradation yielding carbon dioxide, water, hydrochloric and nitric acids (Hustert et al., 1987).
Irradiation of p-chloronitrobenzene in air and nitrogen produced 4-chloro-2-nitrophenol and 4-
chlorophenol, respectively (Kanno and Nojima, 1979).
Chemical. Although no products were identified, p-chloronitrobenzene (1.5 x 10-5 M) was
reduced by iron metal (33.3 g/L acid washed 18–20 mesh) in a carbonate buffer (1.5 x 10-2 M) at
pH 5.9 and 15 °C. Based on the pseudo-first-order disappearance rate of 0.0336/min, the half-life
was 20.6 min (Agrawal and Tratnyek, 1996).
Shipping
UN1578 Chloronitrobenzenes, solid or liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise the nitrobenzene from 95% EtOH (charcoal) and sublime it in vacuo. [Emmons JAm Chem Soc 76 3470 1954, Newman & Forrest J Am Chem Soc 69 1221 1947, Beilstein 5 IV 723.]
Incompatibilities
A strong oxidizer. Reacts violently with oxidizers, combustibles, alkalis, sodium methoxide; and reducing materials.
Waste Disposal
Incineration (816℃, 0.5 second for primary combustion; 1204℃, 1.0 second for secondary combustion). The formation of elemental chlorine can be prevented through injection of steam or methane into the combustion process. nitrogen oxides may be abated through the use of thermal or catalytic devices.
Properties of 4-Chloronitrobenzene
| Melting point: | 80-83 °C(lit.) |
| Boiling point: | 242 °C(lit.) |
| Density | 1.298 g/mL at 25 °C(lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | 0.09 mm Hg ( 25 °C) |
| refractive index | 1.5376 (estimate) |
| Flash point: | >230 °F |
| solubility | Soluble in acetone and alcohol (Weast, 1986) |
| form | Crystals or Flakes |
| color | Yellow |
| Water Solubility | Insoluble |
| Merck | 14,2151 |
| BRN | 508691 |
| Henry's Law Constant | 4.90 x 10-6 atm?m3/mol at 25 °C (thermodynamic method-GC/UV spectrophotometry, Altschuh et al., 1999) |
| Exposure limits | Potential occupational carcinogen. NIOSH REL: IDLH 100; OSHA
PEL: TWA 1. |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, hydroxides. Reacts violently with sodium methoxide in methanol. |
| CAS DataBase Reference | 100-00-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-4-nitro-(100-00-5) |
| IARC | 2B (Vol. 65, 123) 2020 |
| EPA Substance Registry System | p-Chloronitrobenzene (100-00-5) |
Safety information for 4-Chloronitrobenzene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 4-Chloronitrobenzene
| InChIKey | CZGCEKJOLUNIFY-UHFFFAOYSA-N |
4-Chloronitrobenzene manufacturer
Hemani Global
Cns Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 p-Chloronitrobenzene CAS 100-00-5View Details
p-Chloronitrobenzene CAS 100-00-5View Details
100-00-5 -
 1-CHLORO-4-NITROBENZENE For Synthesis CAS 100-00-5View Details
1-CHLORO-4-NITROBENZENE For Synthesis CAS 100-00-5View Details
100-00-5 -
 1-Chloro-4-nitrobenzene CAS 100-00-5View Details
1-Chloro-4-nitrobenzene CAS 100-00-5View Details
100-00-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
