4-Methoxyindole
- CAS NO.:4837-90-5
- Empirical Formula: C9H9NO
- Molecular Weight: 147.17
- MDL number: MFCD00009737
- EINECS: 622-799-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
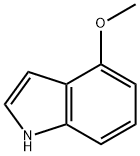
What is 4-Methoxyindole?
Chemical properties
light brown to grey crystalline powder
The Uses of 4-Methoxyindole
4-Methoxyindole is a reactant for alpha-ethyltryptamines as dual dopamine-serotonin releasers.
What are the applications of Application
Reactant for preparation of:
GABA analogs
Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
Anticancer agents
Integrase strand-transfer inhibitors (INSTIs)
Inhibitor of Proliferation of Colon Cancer Cells
Isomeridianin G as GSK-3? inhibitors
HIV-1 integrase inhibitors
Inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2)
4-Methoxyindole was used for comparing the complexation reaction of β-cyclodextrin (β-CD) with pindolol using reversed-phase liquid chromatography.
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 24, p. 1499, 1987 DOI: 10.1002/jhet.5570240602
Biotechnological Applications
4-Methoxyindole is used in a variety of preparation procedures. It is a reactant involved in the synthesis of GABA analogs, HIV-1 integrase inhibitors, anticancer agents, sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors, integrase strand-transfer inhibitors (INSTIs), and isomeridianin G. Additionally, it is used in reverse-phase liquid chromatography analysis of the reaction of β-cyclodextrin and pindolol.
Synthesis
Step1 :1-[(E)-2-(2-methoxy-6-nitrophenyl)vinyl]pyrrolidine: To a solution of 10 g of 1-methoxy-2-methy3-nitrobenzene in 100 ml of DMF are added 8.74 ml of N,N-dimethylformamide dimethyl acetal and 5.44 ml of pyrrolidine, and the mixture is then refluxed for 3 hours. The mixture is concentrated to half its volume under vacuum, the remaining mixture is poured into ether/water and extracted with ether, the organic phase is washed with saturated NaCl solution and dried over MgSO4 , and the solvent is evaporated off under vacuum. 1-[(E)-2-(2-methoxy-6-nitrophenyl)vinyl]pyrrolidine; yield 14.6 g.
Stpe 2 : synthesis of 4-Methoxyindole, A suspension of 150 ml of zinc powder in 500 ml of 0.5N HCl is stirred for 1 hour at room temperature. The suspension is ded by suction, washed with water to neutral pH, with anhydrous EtOH and then with ether, and dried. To a solution of 10 g of the compound from the preceding step in 46 ml of acetic acid are added, portionwise, 31.6 g of activated zinc, while keeping the temperature between 20 and 30°C using an ice bath. The reaction mixture is stirred at room temperature for 30 minutes, and is filtered. The filtrate is extracted with EtOAc, the organic phase is washed with NaHCO3 solution and with saturated NaCl solution, and dried over MgSO4 , and the solvent is evaporated off under vacuum. The residue is chromatographed on silica gel, eluting with a cyclohexane/EtOAc mixture in a gradient of from (98/2 v/v) to (95/5 v/v). 4-Methoxy- 1H-indole; yield 1.6 g.
Properties of 4-Methoxyindole
| Melting point: | 69-70 °C (lit.) |
| Boiling point: | 181-183 °C/24 mmHg (lit.) |
| Density | 1.1135 (rough estimate) |
| refractive index | 1.5310 (estimate) |
| Flash point: | 181-183°C/24mm |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | ethanol: soluble50mg/mL, clear, faintly yellow |
| pka | 17.22±0.30(Predicted) |
| form | Crystalline Powder and/or Chunks |
| color | White |
| Sensitive | Light Sensitive |
| BRN | 122456 |
| CAS DataBase Reference | 4837-90-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-Indole, 4-methoxy-(4837-90-5) |
Safety information for 4-Methoxyindole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Methoxyindole
| InChIKey | LUNOXNMCFPFPMO-UHFFFAOYSA-N |
4-Methoxyindole manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 4-Methoxyindole CAS 4837-90-5View Details
4-Methoxyindole CAS 4837-90-5View Details
4837-90-5 -
 4-Methoxyindole CAS 4837-90-5View Details
4-Methoxyindole CAS 4837-90-5View Details
4837-90-5 -
 4-Methoxyindole 98%View Details
4-Methoxyindole 98%View Details
4837-90-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
