4-Methoxybenzoyl chloride
Synonym(s):p-Anisoyl chloride;4-Anisoyl chloride
- CAS NO.:100-07-2
- Empirical Formula: C8H7ClO2
- Molecular Weight: 170.59
- MDL number: MFCD00000687
- EINECS: 202-816-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-15 13:50:35
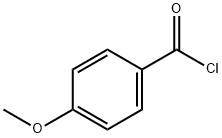
What is 4-Methoxybenzoyl chloride?
Chemical properties
4-methoxybenzoyl chloride appears as an amber-colored crystalline solid. Melting point 72 °F. Corrosive to metals and skin. Vapors may cause serious burns to the eyes. Decompose with water or alcohol, soluble in acetone and benzene.
Definition
The hydrolysis of 4-Methoxybenzoyl chloride (OMe) generally occurs through a dissociative mechanism (SN1). The reaction rate is strongly affected by the polarity of the medium and the ability to stabilize the leaving group. Water encapsulated within a reverse micellar system is less polar and less available compared to a continuous aqueous phase; Consequently, the hydrolysis of OMe in the micellar medium is slower than in pure water.
The Uses of 4-Methoxybenzoyl chloride
4-Methoxybenzoyl chloride is used in the synthesis of stilbene and dihydrostilbene derivatives as potential anti-cancer agents. It is also used in the synthesis of coumarin dimers with potential HIV-1 activity.
What are the applications of Application
4-Methoxybenzoyl chloride is one of the reactive acylating agents that can react with carboxylic acids, alcohols and amines to yield respective carboxylic anhydrides, esters and amides.
4-Methoxybenzoyl chloride can be used as radical precursor in visible-light photocatalysis to synthesize various heterocyclic compounds.
It can be used to synthesize acylphosphine ligands for the rhodium-catalyzed hydrosilylation of alkenes.
Incorporation of 4-methoxybenzoyl chloride modified indium tin oxide (ITO) as cathode for the fabrication of organic light-emitting diodes (OLEDs) has been reported.
1,3 diketones synthesized from 4-methoxybenzoyl chloride can be used in one pot synthesis of various pyrazole derivatives.
It can also be used in the total synthesis of bioactive compounds like echinoside A and salinosporamide A.
Synthesis
4-Methoxybenzoyl chloride is synthesized from the direct chlorination of 4-methoxybenzoic acid by thionyl chloride.
General Description
4-methoxybenzoyl chloride appears as an amber-colored crystalline solid. Melting point 72°F. Corrosive to metals and skin. Vapors may cause serious burns to the eyes.
Air & Water Reactions
Fumes in air. Reacts exothermically with water (including moisture in air or soil) to form hydrochloric acid and insoluble anisic acid [Merck 11th ed. 1989].
Reactivity Profile
ANISOYL CHLORIDE reacts exothermically with bases, including amines. Incompatible with water, strong oxidizing agents, alcohols. Sealed containers held at room temperature may explode, due to slow decomposition that builds up pressure. This situation is more dangerous with heat. May react vigorously or explosively with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Solutions corrosive to tissue. Explosion risk when in closed containers due to pressure caused by decomposition at room temperature.
Health Hazard
Vapor irritates mucous membranes. Contact of liquid with eyes or skin causes severe irritation. Ingestion causes severe irritation of mouth and stomach.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride fumes may be formed.
Flammability and Explosibility
Not classified
Chemical Reactivity
Reactivity with Water Reacts slowly to generate hydrogen chloride (hydrochloric acid). The reaction is not hazardous; Reactivity with Common Materials: Corrodes metal slowly; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Flush with water, rinse with sodium bicarbonate or lime solution; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Safety Profile
Corrosive to skin, eyes, mucous membranes, and other tissue.Evolves HCl by hydrolysis. A storage hazard; can explode spontaneously at room temperature. When heated to decomposition it emits toxic fumes of Cland may explode.
Storage
Stored in a closed container can cause an explosion due to the pressure caused by decomposition, should be stored at low temperature (5°C).
Structure and conformation
Anisoyl chloride, also known as methoxybenzoyl chloride), is an acyl halide, specifically an aromatic acyl chloride, and may be formed from anisic acid by replacing a hydroxyl group of the carboxylic acid with a chloride group. There are three isomers: the ortho-, meta-, and para- forms. 4-Methoxybenzoyl chloride is para- forms. Their structures differ in the arene substitution pattern—the location of the methoxy group on the ring as compared to the acyl halide.
Properties of 4-Methoxybenzoyl chloride
| Melting point: | 22 °C(lit.) |
| Boiling point: | 262-263 °C(lit.) |
| Density | 1.260 g/mL at 20 °C(lit.) |
| vapor pressure | 1.853Pa at 25℃ |
| refractive index | n |
| Flash point: | 190 °F |
| storage temp. | 2-8°C |
| form | Liquid After Melting |
| color | Clear colorless to brown |
| Odor | Sharp, penetrating. |
| Water Solubility | It reacts in water. |
| Sensitive | Moisture Sensitive |
| Merck | 14,673 |
| BRN | 471918 |
| Stability: | Stable, but reacts violently with water. Ensure no moisture enters the container in which this material is stored, to prevent pressure build-up. Incompatible with water, moisture, strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 100-07-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Methoxybenzoic acid chloride(100-07-2) |
| EPA Substance Registry System | Benzoyl chloride, 4-methoxy- (100-07-2) |
Safety information for 4-Methoxybenzoyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 4-Methoxybenzoyl chloride
| InChIKey | MXMOTZIXVICDSD-UHFFFAOYSA-N |
4-Methoxybenzoyl chloride manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 "4-METHOXYBENZOYL CHLORIDE " 100-07-2 98%View Details
"4-METHOXYBENZOYL CHLORIDE " 100-07-2 98%View Details
100-07-2 -
 Anisoyl chloride 98%View Details
Anisoyl chloride 98%View Details -
 4-Methoxybenzoyl chloride, 97% 99%View Details
4-Methoxybenzoyl chloride, 97% 99%View Details
100-07-2 -
 4-Methoxybenzoyl Chloride CAS 100-07-2View Details
4-Methoxybenzoyl Chloride CAS 100-07-2View Details
100-07-2 -
 4-Methoxybenzoyl chloride CAS 100-07-2View Details
4-Methoxybenzoyl chloride CAS 100-07-2View Details
100-07-2 -
 4-Methoxybenzoyl chloride CAS 100-07-2View Details
4-Methoxybenzoyl chloride CAS 100-07-2View Details
100-07-2 -
 4-METHOXY BENZOYL CHLORIDE Cas no 100-07-4, 99%, Industrial GradeView Details
4-METHOXY BENZOYL CHLORIDE Cas no 100-07-4, 99%, Industrial GradeView Details
100-07-2 -
 4-Anisoyl Chloride Cas No 100-07-2View Details
4-Anisoyl Chloride Cas No 100-07-2View Details
100-07-2
