4-Formylphenylboronic acid
Synonym(s):p-Formylbenzeneboronic acid;p-Formylphenylboronic acid;4-(Dihydroxyboryl)benzaldehyde;4-Boronobenzaldehyde;4-Formylbenzeneboronic acid
- CAS NO.:87199-17-5
- Empirical Formula: C7H7BO3
- Molecular Weight: 149.94
- MDL number: MFCD00151823
- EINECS: 438-670-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
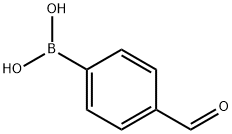
What is 4-Formylphenylboronic acid?
Chemical properties
white to light yellow crystal powder
The Uses of 4-Formylphenylboronic acid
4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions and it can be used as a reagent for:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
The Uses of 4-Formylphenylboronic acid
suzuki reaction
The Uses of 4-Formylphenylboronic acid
4-Formylbenzeneboronic acid acts as a reagent used for Palladium-catalyzed arylation Suzuki-Miyaura cross-coupling in water, copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides, ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids, triethylamine-catalyzed three-component Hantzsch condensations, copper-catalyzed nitrations, oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta, palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides, palladium-catalyzed aerobic oxidative cross-coupling reactions. It acts as a reagent used in preparation of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells, a novel protein synthesis inhibitor active against Gram-positive bacteria.
What are the applications of Application
4-Formylphenylboronic acid is used for various catalytic reactions
Flammability and Explosibility
Not classified
Synthesis
Potassium phenyltrifluoroborate (184 mg, 1.00 mmol) was added to a solution of iron trichloride (185 mg, 1.10 mmol) in 3 mL of 1:1 THF/water. The mixture was stirred at room temperature for 30 min. The reaction mixture was then passed through a short column containing neutral absorption alumina. The alumina was then washed with a mixture of ethyl acetate/hexanes (2:1) to obtain 4-Formylphenylboronic acid (105 mg, 86%). [The boronic acid products can also be isolated by simple extraction techniques.]
Properties of 4-Formylphenylboronic acid
| Melting point: | 237-242 °C (lit.) |
| Boiling point: | 347.6±44.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | <10g/l |
| form | Liquid |
| pka | 7.34±0.10(Predicted) |
| color | Clear colorless to yellow-orange |
| PH | 5.5 (1g/l, H2O, 20℃) |
| Water Solubility | Slightly Soluble in water. |
| Sensitive | Air Sensitive |
| BRN | 3030770 |
| CAS DataBase Reference | 87199-17-5(CAS DataBase Reference) |
| EPA Substance Registry System | Boronic acid, (4-formylphenyl)- (87199-17-5) |
Safety information for 4-Formylphenylboronic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for 4-Formylphenylboronic acid
| InChIKey | VXWBQOJISHAKKM-UHFFFAOYSA-N |
4-Formylphenylboronic acid manufacturer
JSK Chemicals
Malladi Drugs AND Pharmaceuticals Limited
Rivashaa Agrotech Biopharma Pvt. Ltd.
Dr R R Organics
Denisco Chemicals Pvt Ltd
Prabhat Chemiorganics Limited
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 4-FORMYL PHENYL BORONIC ACID 87199-17-5 98%View Details
4-FORMYL PHENYL BORONIC ACID 87199-17-5 98%View Details
87199-17-5 -
 87199-17-5 4-Formylphenylboronic acid, 95% 99%View Details
87199-17-5 4-Formylphenylboronic acid, 95% 99%View Details
87199-17-5 -
 4-Formylphenylboronic Acid extrapure CAS 87199-17-5View Details
4-Formylphenylboronic Acid extrapure CAS 87199-17-5View Details
87199-17-5 -
 4-Formylphenylboronic Acid (contains varying amounts of Anhydride) CAS 87199-17-5View Details
4-Formylphenylboronic Acid (contains varying amounts of Anhydride) CAS 87199-17-5View Details
87199-17-5 -
 4-(Formylphenyl) boronic acid 87199-17-5 98%View Details
4-(Formylphenyl) boronic acid 87199-17-5 98%View Details
87199-17-5 -
 87199-17-5 99%View Details
87199-17-5 99%View Details
87199-17-5 -
 4-Formylphenylboronic acid, 97% CAS 87199-17-5View Details
4-Formylphenylboronic acid, 97% CAS 87199-17-5View Details
87199-17-5 -
 4-Formylphenylboronic acid CAS 87199-17-5View Details
4-Formylphenylboronic acid CAS 87199-17-5View Details
87199-17-5