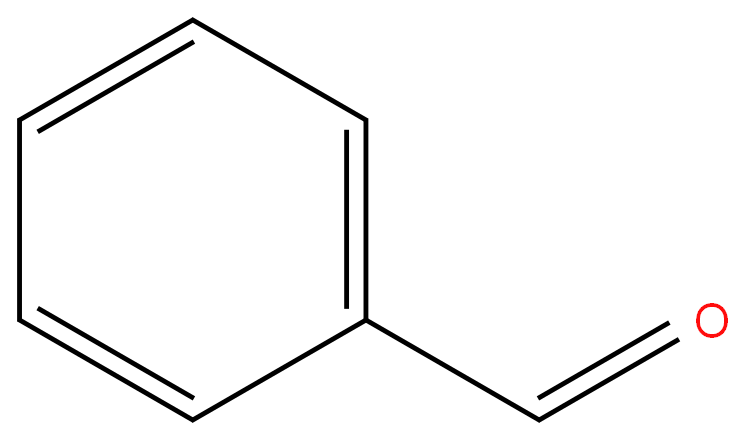
Benzaldehyde
Synonym(s):Benzaldehyde;Bitter almond;Bitter almond oil
- CAS NO.:100-52-7
- Empirical Formula: C7H6O
- Molecular Weight: 106.12
- MDL number: MFCD00003299
- EINECS: 202-860-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-20 19:16:41
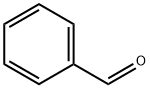
What is Benzaldehyde?
Description
Benzaldehyde is a clear to yellowish liquidwith an almond odor. The odor threshold is 0.042 ppm.Molecular weight=106.13; Specific gravity (H2O:1)=1.1;Boiling point=179℃; Freezing/Melting point=2 56℃;Flash point=63℃ (cc); Vapor pressure=0.99 mmHg at20℃; Autoignition temperature=190℃. Explosive limits:LEL=1.4%, UEL=13.5%. Hazard Identification (basedon NFPA-704 M Rating System): Health 2, Flammability 2,Reactivity 1. Practically insoluble in water;solubility=0.3 g/100 mL
Chemical properties
Benzaldehyde is a colorless to yellow, oily liquid with an odor of bitter almonds. Benzaldehyde is commercially available in two grades: (i) pure benzaldehyde and (ii) and double-distilled benzaldehyde. The latter has applications in the pharmaceutical, perfume, and fl avor industries. Benzaldehyde may contain trace amounts of chlorine, water, benzoic acid, benzyl chloride, benzyl alcohol, and/or nitrobenzene. Benzaldehyde is ignited relatively easily on contact with hot surfaces. This has been attributed to the property of very low auto-ignition temperature. Benzaldehyde also undergoes autoxidation in air and is liable to self-heat. It is used as a food flavoring and in the manufacture of dyes and antibiotics, and can be readily manufactured by the chlorination of methylbenzene and the subsequent hydrolysis of (dichloromethyl) benzene: C6H5CH3 + Cl2 →C6H5CHCl2C6H5CHCl2 + 2H2O →C6H5CH(OH)2+ 2HCl C6H5CH(OH)2 →C6H5CHO + H2O.
Occurrence
Benzaldehyde exists in nature, occurring in combined and uncombined forms in many plants. It is also the main constituent of the essential oils obtained by pressing the kernels of peaches, cherries, apricots, and other fruits. Benzaldehyde is released into the environment in emissions from combustion processes, such as gasoline and diesel engines, incinerators, and wood burning. It is formed in the atmosphere through photochemical oxidation of toluene and other aromatic hydrocarbons.
The Uses of Benzaldehyde
Benzaldehyde is a flavoring agent which is liquid and colorless, and has an almond-like odor. it has a hot (burning) taste. it is oxidized to benzoic acid when exposed to air and deteriorates under light. it is miscible in volatile oils, fixed oils, ether, and alcohol; it is spar- ingly soluble in water. it is obtained by chemical synthesis and by natural occurrence in oils of bitter almond, peach, and apricot kernel. it is also termed benzoic aldehyde.
The Uses of Benzaldehyde
Manufacture of dyes, perfumery, cinnamic and mandelic acids, as solvent; in flavors.
The Uses of Benzaldehyde
Benzaldehyde is used as an intermediatein the production of flavoring chemicals,such as cinnamaldehyde, cinnamalalcohol,and amyl- and hexylcinnamaldehyde for perfume,soap, and food flavor; synthetic penicillin,ampicillin, and ephedrine; and as araw material for the herbicide Avenge. Itoccurs in nature in the seeds of almonds,apricots, cherries, and peaches. It occurs intrace amounts in corn oil.
Definition
ChEBI: Benzaldehyde is an arenecarbaldehyde that consists of benzene bearing a single formyl substituent; the simplest aromatic aldehyde and parent of the class of benzaldehydes. It has a role as a flavouring agent, a fragrance, an odorant receptor agonist, a plant metabolite, an EC 3.5.5.1 (nitrilase) inhibitor and an EC 3.1.1.3 (triacylglycerol lipase) inhibitor.
Preparation
Benzaldehyde is prepared by hydrolysis of benzal chloride, for example, in acidic media in the presence of a catalyst such as ferric chloride or in alkaline media with aqueous sodium carbonate. Part of the commercially available benzaldehyde originates from a technical process for phenol. In this process, benzaldehyde is a by-product in the oxidation, in air, of toluene to benzoic acid.
Reactions
Benzaldehyde reacts with many chemicals in a marked manner: (1) with ammonio-silver nitrate (“Tollen’s solution”) to form metallic silver, either as a black precipitate or as an adherent mirror film on glass (but does not reduce alkaline cupric solution, “Fehling’s solution”); (2) with rosaniline (fuchsine, magenta) that has been decolorized by sulfurous acid (“Schiff’s solution”), restoring the pink color of rosaniline; (3) with NaOH solution, yielding benzyl alcohol and sodium benzoate; (4) with NH4OH, yielding tribenzaldeamine (hydrobenzamide, (C6H5CH)3N2), white solid, mp 101 °C, (5) with aniline, yielding benzylideneaniline (“Schiff’s base” C6H5CH:NC6H5); (6) with sodium cyanide in alcohol, yielding benzoin C6H5·CHOHCOC6H5, white solid, mp 133 °C; (7) with hydroxylamine hydrochloride, yielding benzaldoximes C6H5CH:NOH, white solids, antioxime, mp 35 °C, syn-oxime, mp 130 °C; (8) with phenylhydrazine, yields benzaldehyde phenylhydrazone C6H5CH:NNHC6H5, pink solid, mp 156 °C; (9) with concentrated HNO3, yields metanitrobenzaldehyde NO2·C6H4CHO, white solid, mp 58 °C; (10) with concentrated H2SO4 yields metabenzaldehyde sulfonic acid C6H4CHO (SO3H)2, (11) with anhydrous sodium acetate and acetic anhydride at 180 °C, yielding sodium benzoate C6H5CHOONa (12) with sodium hydrogen sulfite, forming benzaldehyde sodium bisulfite C6H5CHOHSO3Na, a white solid, from which benzaldehyde is readily recoverable by treatment with sodium carbonate solution; (13) with acetaldehyde made slightly alkaline with NaOH, yielding cinnamic aldehyde C6H5CH:CHCHO, (14) with phosphorus pentachloride, yielding benzylidine chloride C6H5CHCl2.
Aroma threshold values
Detection: 100 ppb to 4.6 ppm; Recognition: 330 ppb to 4.1 ppm.
Taste threshold values
Taste characteristics at 50 ppm: sweet, oily, almond, cherry, nutty and woody
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 12, p. 403, 1964
The Journal of Organic Chemistry, 58, p. 4732, 1993 DOI: 10.1021/jo00069a043
Synthetic Communications, 16, p. 43, 1986 DOI: 10.1080/00397918608057686
General Description
Benzaldehyde appears as a clear colorless to yellow liquid with a bitter almond odor. Flash point near 145 °F. More denser than water and insoluble in water. Hence sinks in water. Vapors are heavier than air. The primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways. Used in flavoring and perfume making.
Air & Water Reactions
Oxidizes in air to form benzoic acid, which is moderately toxic by ingestion. Insoluble in water.
Reactivity Profile
A nontoxic, combustible liquid, reacts with oxidizing reagents. Benzaldehyde must be blanketed with an inert gas at all times since Benzaldehyde is oxidized readily by air to benzoic acid [Kirk-Othmer, 3rd ed., Vol. 3, 1978, p. 736]. In contact with strong acids or bases Benzaldehyde will undergo an exothermic condensation reaction [Sax, 9th ed., 1996, p. 327]. A violent reaction was observed on contact with peroxyacids (peroxyformic acid) [DiAns, J. et al., Ber., 1915, 48, p. 1136]. An explosion occurred when pyrrolidine, Benzaldehyde, and propionic acid were heated to form porphyrins.
Hazard
Highly toxic.
Health Hazard
Benzaldehyde exhibited low to moderate toxicityin test animals, the poisoning effectdepending on dosage. Ingestion of 50–60 mLmay be fatal to humans. Oral intake of a largedose can cause tremor, gastrointestinal pain,and kidney damage. Animal experimentsindicated that ingestion of this compoundby guinea pigs caused tremor, bleeding fromsmall intestine, and an increase in urine volume;in rats, ingestion resulted in somnolenceand coma.
LD50 value, oral (guinea pigs): 1000 mg/kg
LD50 value, oral (rats): 1300 mg/kg
A 500-mg amount for a 24-hour periodresulted in moderate skin irritation in rabbits.Because of its low toxicity, high boilingpoint, and low vapor pressure, the healthhazard to humans from exposure to benzaldehydeis very low.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water: No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Pharmacology
Benzaldehyde significantly inhibited peptic activity in artificial gastric juice in vitro
(20-45% inhibition) and in vivo to the extent of 87% in normal healthy persons and ulcer patients
(Kleeberg, 1959). As a freshly prepared 1:500 solution, it exerted a marked antispasmodic effect,
relaxing the tonus and inhibiting contractions of various isolated smooth muscles of dog, cat, rat,
rabbit, mouse, guinea-pig, pig and frog and of a few human tissues. Injected into rabbits and other
animals it produced a marked relaxation of the intestines and urinary bladder and marked vasodilation
of the splanchnic vessel. Injection of 4 ml of a 5% solution iv into a cat caused a fall in
blood pressure and slowing of respiration. In dogs, 1 ml injected iv or sc or 2 ml/kg given orally
produced only a slight slowing of respiration. Injection of larger doses iv produced only a drop
in blood pressure, slight slowing of respiration and inhibition of intestinal contractions, with vasodilation
of the splanchnic vessel. In rabbits, iv injection of 20 ml of a 0-2% solution did not produce
dangerous results. Large injected doses of benzaldehyde exert their mosjt important toxic effects
on the medulla, with slowing or paralysis of respiration. In the intact animal, the heart is very
little affected; but benzaldehyde acts as a muscular depressant on isolated frog heart (Macht, 1922).
Treatment of isolated rat striated muscle for 1-5 min with 30 mM-benzaldehyde increased the
rate of propagation of contractures and the rate of structural breakdown of injured striated muscle
fibres. After more prolonged application (for 30 min), the rapid propagation of contracture continued
but the structural breakdown was inhibited (Busing, 1972).
Benzaldehyde possessed definite local anaesthetic properties in the sciatic nerves of cats, dogs
and frogs, in the eyes of rabbits and dogs (accompanied by irritation) and in the skin of frogs,
but was considered unsuitable for practical use because of its rapid oxidation to benzoic acid (Macht,
1922).
In a study of the toxic effects of cherry laurel water on mice and on isolated rat intestine, benzaldehyde
was found to aid in the detoxication of HCN by the formation of C6H5?CH(OH)?CN (Lanza
& Conte, 1964).
Benzaldehyde did not act as a cross-linking (tanning) agent for corium and aorta, since in a
015 M solution it did not increase the observed in vitro hydrothermal shrinkage temperatures of
goat skin and human, bovine and canine aortae (Milch, 1965).
The intestinal absorption-rate coefficients of benzaldehyde and related compounds were determined
by perfusion of aqueous solutions through the small intestines of anaesthetized rats (Nogami, Hanano
& Yamada, 1968).
No changes in gastric motor patterns, including gastric motility, were observed in rats after inhalation
of "toxic levels" (not specified) of benzaldehyde from a liquid sample placed in a test chamber
using recirculated air, or from a saturated paper applied to the trachea (Roth & Tansy, 1972).
Benzaldehyde in a concentration of 0-1 mmol/litre caused a 16% depression of the frequency of
electric-organ discharge in the mormyrid electric fish Gnathonemus moori (Walsh & Schopp, 1966).
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by subcutaneous route. An allergen. Acts as a feeble local anesthetic. Local contact may cause contact dermatitis. Causes central nervous system depression in small doses and convulsions in larger doses. A skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible liquid. To fight fire, use water (may be used as a blanket), alcohol, foam, dry chemical. A strong reducing agent. Reacts violently with peroxyformic acid and other oxidizers. See also ALDEHYDES.
Synthesis
Natural benzaldehyde is obtained by extraction and subsequent fractional distillation from botanical sources; synthetically, from benzyl chloride and lime or by oxidation of toluene
Potential Exposure
In manufacture of perfumes, dyes, and cinnamic acid; as solvent; in flavors.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Metabolism
Benzaldehyde was among 300 volatile constituents detected in the urine of ten adults . It is commonly converted to hippuric acid in vivo. In the rabbit and dog, hippuric acid appears to be the only metabolite there being practically no formation of benzoyl glucuronide. The conversion of benzaldehyde to benzoic acid in the rabbit follows first-order reaction kinetics
storage
Benzaldehyde should be kept stored in a tightly closed container and protected against physical damage. Storage of the chemical substance outside or in a detached area is preferred, whereas inside storage should be in a standard flammable liquids storage room or cabinet. Benzaldehyde should be kept separated from oxidizing materials. Also, storage and use areas should be no smoking areas. Containers of this material may be hazardous when empty since they retain product residues (vapors, liquid); observe all warnings and precautions listed for the product
Shipping
UN1990 Benzaldehyde, Hazard class: 9; Labels: 9—Miscellaneous hazardous material.
Purification Methods
To diminish its rate of oxidation, benzaldehyde usually contains additives such as hydroquinone or catechol. It can be purified via its bisulfite addition compound but usually distillation (under nitrogen at reduced pressure) is sufficient. Prior to distillation it is washed with NaOH or 10% Na2CO3 (until no more CO2 is evolved), then with saturated Na2SO3 and H2O, followed by drying with CaSO4, MgSO4 or CaCl2. [Beilstein 7 IV 505.]
Incompatibilities
The substance reacts with air, forming explosive peroxides. Reacts violently with performic acid, oxidants, aluminum, iron, bases, and phenol, causing fire and explosion hazard. May self-ignite if absorbed in combustible material with large surface area, or otherwise dispersed over large areas. Reacts with rust, amines, alkalies, strong bases, reducing agents such as hydrideds and active metals.
Waste Disposal
Incineration; add combustible solvent and spray into incinerator with afterburner.
Precautions
Workers should be careful when using benzaldehyde because there is a risk of spontaneous combustion. It may ignite spontaneously if it is absorbed onto rags, cleaning cloths, clothing, sawdust, diatomaceous earth (kieselguhr), activated charcoal, or other materials with large surface areas in workplaces. Workers should avoid handling the chemical substance and should not cut, puncture, or weld on or near the container. Exposure of benzaldehyde to air, light, heat, hot surfaces such as hot pipes, sparks, open flames, and other ignition sources should be avoided. Workers should wear proper personal protective clothing and equipment
Properties of Benzaldehyde
| Melting point: | -26 °C (lit.) |
| Boiling point: | 178-179 °C (lit.) |
| Density | 1.044 g/cm3 at 20 °C (lit.) |
| vapor density | 3.7 (vs air) |
| vapor pressure | 4 mm Hg ( 45 °C) |
| refractive index | n |
| FEMA | 2127 | BENZALDEHYDE |
| Flash point: | 145 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble100mg/mL |
| form | neat |
| pka | 14.90(at 25℃) |
| color | Pale yellow |
| Odor | Like almonds. |
| PH | 5.9 (1g/l, H2O) |
| PH Range | 5.9 |
| explosive limit | 1.4-8.5%(V) |
| Water Solubility | <0.01 g/100 mL at 19.5 ºC |
| FreezingPoint | -56℃ |
| Sensitive | Air Sensitive |
| Merck | 14,1058 |
| JECFA Number | 22 |
| BRN | 471223 |
| Dielectric constant | 17.8(20℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, reducing agents, steam. Air, light and moisture-sensitive. |
| CAS DataBase Reference | 100-52-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde(100-52-7) |
| EPA Substance Registry System | Benzaldehyde (100-52-7) |
Safety information for Benzaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Benzaldehyde
| InChIKey | HUMNYLRZRPPJDN-UHFFFAOYSA-N |
Benzaldehyde manufacturer
JSK Chemicals
Panorama Aromatics Ltd
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 Benzaldehyde, 99% 100-52-7 98%View Details
Benzaldehyde, 99% 100-52-7 98%View Details
100-52-7 -
 BENZALDEHYDE. 99%View Details
BENZALDEHYDE. 99%View Details -
 Benzaldehyde (SQ) CAS 100-52-7View Details
Benzaldehyde (SQ) CAS 100-52-7View Details
100-52-7 -
 Benzaldehyde extrapure AR CAS 100-52-7View Details
Benzaldehyde extrapure AR CAS 100-52-7View Details
100-52-7 -
 Benzaldehyde CASView Details
Benzaldehyde CASView Details -
 Benzaldehyde 99%View Details
Benzaldehyde 99%View Details -
 Benzaldehyde CAS 100-52-7View Details
Benzaldehyde CAS 100-52-7View Details
100-52-7 -
 Benzaldehyde CAS 100-52-7View Details
Benzaldehyde CAS 100-52-7View Details
100-52-7
