4-Chlorophenyl isocyanate
Synonym(s):4-Isocyanato chlorobenzene
- CAS NO.:104-12-1
- Empirical Formula: C7H4ClNO
- Molecular Weight: 153.57
- MDL number: MFCD00002024
- EINECS: 203-176-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
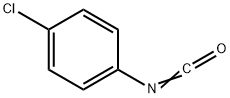
What is 4-Chlorophenyl isocyanate?
Chemical properties
white to yellow crystalline solid
The Uses of 4-Chlorophenyl isocyanate
4-Chlorophenyl isocyanate is used in the preparation of isothiocyanates. Aryl isocyanates undergo [2+2] cycloaddition reactions with dihydrofuran to give bicyclic ?-lactams in high yield.
The Uses of 4-Chlorophenyl isocyanate
4-Chlorophenyl isocyanate was used in the preparation of isothiocyanates.
Synthesis Reference(s)
The Journal of Organic Chemistry, 34, p. 3200, 1969 DOI: 10.1021/jo01262a089
General Description
Colorless to yellow liquid or crystals. Used as an intermediate in pesticide and pharmaceutical manufacture.
Reactivity Profile
Isocyanates and thioisocyanates are incompatible with many classes of compounds, reacting exothermically to release toxic gases. Reactions with amines, aldehydes, alcohols, alkali metals, ketones, mercaptans, strong oxidizers, hydrides, phenols, and peroxides can cause vigorous releases of heat. Acids and bases initiate polymerization reactions in these materials. Some isocyanates react with water to form amines and liberate carbon dioxide. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence, [Wischmeyer(1969)].
Safety Profile
Poison by ingestion and inhalation. Unspecified human systemic effects. A severe eye and moderate skin irritant. A flammable liquid when exposed to heat or flame. Dangerous, can explode on distillation. When heated to decomposition it emits toxic fumes of Cl-, CN-, and NOx.
Purification Methods
Purify the isocyanate by recrystallisation from pet ether (b 30-40o) or better by fractional distillation. TOXIC irritant. [Beilstein 12 H 616, 12 III 1376, 12 IV 1213.]
Properties of 4-Chlorophenyl isocyanate
| Melting point: | 26-29 °C (lit.) |
| Boiling point: | 203-204 °C (lit.) |
| Density | 1.2 g/mL at 25 °C (lit.) |
| vapor pressure | 30Pa at 20℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly) |
| form | Crystalline Solid |
| color | White to yellow |
| Odor | sharp irritating odor |
| Water Solubility | DECOMPOSES |
| Sensitive | Moisture Sensitive/Lachrymatory |
| BRN | 386235 |
| CAS DataBase Reference | 104-12-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-4-isocyanato-(104-12-1) |
| EPA Substance Registry System | p-Chlorophenyl isocyanate (104-12-1) |
Safety information for 4-Chlorophenyl isocyanate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Chlorophenyl isocyanate
4-Chlorophenyl isocyanate manufacturer
Paushak Limited
ALS INDIA LIFE SCIENCES
G C Int'L
ASM Organics
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 104-12-1 4-Chlorophenyl isocyanate-98% 99%View Details
104-12-1 4-Chlorophenyl isocyanate-98% 99%View Details
104-12-1 -
 104-12-1 98%View Details
104-12-1 98%View Details
104-12-1 -
 para-Chlorophenyl Isocyanate 98%View Details
para-Chlorophenyl Isocyanate 98%View Details
104-12-1 -
 para-Chlorophenyl Isocyanate 104-12-1 98%View Details
para-Chlorophenyl Isocyanate 104-12-1 98%View Details
104-12-1 -
 4-Chlorophenyl Isocyanate CAS 104-12-1View Details
4-Chlorophenyl Isocyanate CAS 104-12-1View Details
104-12-1 -
 4-Chlorophenyl isocyanate CAS 104-12-1View Details
4-Chlorophenyl isocyanate CAS 104-12-1View Details
104-12-1 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8