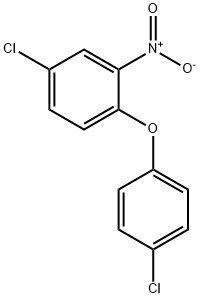
4-Chlorodiphenyl ether
Synonym(s):4-Chlorophenyl phenyl ether
- CAS NO.:7005-72-3
- Empirical Formula: C12H9ClO
- Molecular Weight: 204.65
- MDL number: MFCD00055431
- EINECS: 230-281-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
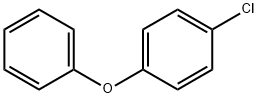
What is 4-Chlorodiphenyl ether?
Chemical properties
colorless to light yellow liquid
The Uses of 4-Chlorodiphenyl ether
4-Chlorodiphenyl Ether is a standard for environmental testing and research. Biodegradability studies with organic priority pollutant compounds.
The Uses of 4-Chlorodiphenyl ether
4-Chlorodiphenyl ether can be used in the preparation of 4′-chloro-2,2′,3,3′,4,5,5′,6,6′-nonabromodiphenyl ether (Cl-BDE-208), an internal standard used in the analysis of highly brominated diphenyl ethers.
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 391, 1992 DOI: 10.1021/jo00027a071
General Description
Liquid. Density 1.193 g / cm3. Insoluble or slightly soluble in water.
Air & Water Reactions
Insoluble or slightly soluble in water.
Reactivity Profile
4-Chlorodiphenyl ether oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164].
Fire Hazard
Combustible.
Environmental Fate
Biological. 4-Chlorophenyl phenyl ether (5 and 10 mg/L) did not significantly biodegrade
following incubation in settled domestic wastewater inoculum at 25 °C. Percent losses reached a
maximum after 2–3 wk but decreased thereafter suggesting a deadaptive process was occurring
(Tabak et al., 1981). In activated sludge, a half-life of 4.0 h was measured (Branson, 1978).
Photolytic. In a methanolic solution irradiated with UV light (λ >290 nm), dechlorination of 4-
chlorophenyl phenyl ether resulted in the formation of diphenyl ether (Choudhry et al., 1977).
Photolysis of an aqueous solution containing 10% acetonitrile with UV light (λ = 230–400 nm)
yielded 4-hydroxybiphenyl ether and chloride ion (Dulin et al., 1986).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities
were 111, 61, 33, and 18 mg/g, respectively (Dobbs and Cohen, 1980).
Properties of 4-Chlorodiphenyl ether
| Melting point: | -8 °C |
| Boiling point: | 161-162 °C/19 mmHg (lit.) |
| Density | 1.193 g/mL at 25 °C (lit.) |
| vapor pressure | 2.7 x 10-3 mmHg at 25 °C (calculated, Branson, 1977) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Refrigerator, under inert atmosphere |
| solubility | 3.3 mg/L at 25 °C (Branson, 1977) |
| form | Liquid |
| color | Colourless |
| Specific Gravity | 1.193 |
| Water Solubility | 3.3 mg/L (25 ºC) |
| Henry's Law Constant | 2.2 x 10-4 atm?m3/mol(Pankow and Rosen, 1988) |
| CAS DataBase Reference | 7005-72-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-4-phenoxy-(7005-72-3) |
| EPA Substance Registry System | p-Chlorophenyl phenyl ether (7005-72-3) |
Safety information for 4-Chlorodiphenyl ether
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 4-Chlorodiphenyl ether
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 4-Chlorodiphenyl ether CAS 7005-72-3View Details
4-Chlorodiphenyl ether CAS 7005-72-3View Details
7005-72-3 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1