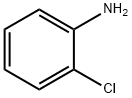
4-Chloroaniline
Synonym(s):4-Chloroaniline;p-Chloroaniline
- CAS NO.:106-47-8
- Empirical Formula: C6H6ClN
- Molecular Weight: 127.57
- MDL number: MFCD00007835
- EINECS: 203-401-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
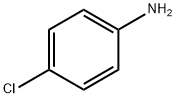
What is 4-Chloroaniline?
Description
p-Chloroaniline, 1-amino-4- chlorobenzene,is a white to light amber, crystalline solid at room temperature. It is used as an intermediate for pesticides, pharmaceuticals, pigments, and dyes. Production is similar to that of m-chloroaniline .
Chemical properties
4-Chloroaniline is a colourless to slightly amber-coloured crystalline solid with a mild aromatic odour. It is Soluble in hot water and organic solvents. 4-Chloroaniline has a moderate vapour pressure and n-octanol/ water partition coefficient. It decomposes in the presence of light and air and at elevated temperatures.
Physical properties
Yellowish-white solid with a mild, sweetish odor. Odor threshold concentration is 287 ppm (quoted, Keith and Walters, 1992).
The Uses of 4-Chloroaniline
4-Chloroaniline is an important raw material in production of agricultural chemicals, azo dyes and pigments, cosmetics, and pharmaceutical products. It is used as an intermediate in the manufacture of chromophore AS-LB, as well as in pharmaceutical intermediates such as chlordiazepoxide and phena tincture. It is also an intermediate for the herbicide Anilofos, the insecticide chlorbenzuron and the plant growth regulator Inabenfide.
Definition
ChEBI: 4-chloroaniline is a chloroaniline in which the chloro atom is para to the aniline amino group. It is a chloroaniline and a member of monochlorobenzenes.
What are the applications of Application
4-Chloroaniline is an important building block used in the chemical industry for the production of drugs and dyestuffs. Some benzodiazepine drugs use 4-chloroaniline in their manufacture.
Preparation
synthesis of 4-Chloroaniline: p-chloronitrobenzene is used as raw material, Raney nickel is used as catalyst, ethanol is used as solvent, reaction temperature is 50~70°C , hydrogenation pressure is 3.04~3.55MPa, and medium pH=5~6 conditions, carry out Catalytic hydrogenation reaction to obtain 4-Chloroaniline.
Synthesis Reference(s)
Journal of the American Chemical Society, 99, p. 98, 1977 DOI: 10.1021/ja00443a018
Synthesis, p. 48, 1987 DOI: 10.1055/s-1987-27838
General Description
P-chloroaniline appears as a white or pale yellow solid. Melting point 69.5°C.
Air & Water Reactions
Insoluble in cold water. Soluble in hot water [Hawley].
Reactivity Profile
4-Chloroaniline is incompatible with oxidizing agents. Also incompatible with acids, acid chlorides, acid anhydrides and chloroformates. Subject to exothermic decomposition during high-temperature distillation. Incompatible with nitrous acid.
Hazard
Toxic by inhalation and ingestion. Possible carcinogen.
Health Hazard
Inhalation or ingestion causes bluish tint to fingernails, lips, and ears indicative of cyanosis; headache, drowsiness, and nausea, followed by unconsciousness. Liquid can be absorbed through skin and cause similar symptoms. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating and toxic hydrogen chloride and oxides of nitrogen may form in fires.
Flammability and Explosibility
Flammable
Safety Profile
Confirmed carcinogen with experimental neoplastigenic and tumorigenic data. Poison by ingestion, inhalation, sh contact, subcutaneous, and intravenous routes. A skin and severe eye irritant. Mutation data reported. When heated to decomposition it emits toxic fumes of Cland NOx. See also ANILINE DYES
Environmental Fate
Biological. In an anaerobic medium, the bacteria of the Paracoccus sp. converted 4-
chloroaniline to 1,3-bis(p-chlorophenyl)triazene and 4-chloroacetanilide with product yields of 80
and 5%, respectively (Minard et al., 1977). In a field experiment, [14C]4-chloroaniline was applied
to a soil at a depth of 10 cm. After 20 wk, 32.4% of the applied amount was recovered in soil.
Metabolites identified include 4-chloroformanilide, 4-chloroacetanilide, 4-chloronitrobenzene, 4-
chloronitrosobenzene, 4,4′-dichloroazoxybenzene, and 4,4′-dichloroazobenzene (Freitag et al.,
1984).
Soil. 4-Chloroaniline covalently bonds with humates in soils to form quinoidal structures
followed by oxidation to yield a nitrogen-substituted quinoid ring. A reaction half-life of 13 min
was determined with one humic compound (Parris, 1980). Catechol, a humic acid monomer,
reacted with 4-chloroaniline yielding 4,5-bis(4-chlorophenylamino)-3,5-cyclohexadiene-1,2-dione
(Adrian et al., 1989).
Photolytic. Under artificial sunlight, river water containing 2–5 ppm 4-chloroaniline
photodegraded to 4-aminophenol and unidentified polymers (Mansour et al., 1989).
Photooxidation of 4-chloroaniline (100 μM) in air-saturated water using UV light (λ >290 nm)
produced 4-chloronitrobenzene and 4-chloronitrosobenzene. About 6 h later, 4-chloroaniline
completely reacted leaving dark purple condensation products (Miller and Crosby, 1983). In a
similar study, irradiation of an aqueous solution in the range of 290–350 nm resulted in the
formation of the intermediate 4-iminocyclohexa-2,5-dienylidene (Othmen et al., 2000). A carbon
dioxide yield of 27.7% was achieved when 4-chloroaniline adsorbed on silica gel was irradiated
with light (λ >290 nm) for 17 h (Freitag et al., 1985).
A rate constant of 8.3 x 10-11 cm3/molecule?sec was reported for the gas-phase reaction of 4-
chloroaniline and OH radicals in air (Wahner and Zetzsch, 1983).
Chemical/Physical. 4-Chloroaniline will not hydrolyze to any reasonable extent (Kollig, 1993).
Pizzigallo et al. (1998) investigated the reaction of 4-chloroaniline with ferric oxide and two
forms of manganese dioxide [birnessite (δ-MnO2) and pyrolusite (MnO2)] within the pH range of
4–8 at 25 °C. The reaction rate of 4-chloroaniline was in the order birnessite > pyrolusite > ferric
oxide. At pH 4.0, the reaction with birnessite was so rapid that the reaction could not be
determined. Half-lives for the reaction of 4-chloroaniline with pyrolusite and ferric oxide were 383
and 746 min, respectively. The reaction rate decreased as the pH was increased. The only
oxidation compounds identified by GC/MS were 4,4′-dichloroazobenzene and 4-chloro-4′-
hydroxydiphenylamine.
Purification Methods
Crystallise the aniline from MeOH, pet ether (b 30-60o), or 50% aqueous EtOH, then *benzene/pet ether (b 60-70o), and then dry it in a vacuum desiccator. It can be distilled under vacuum (b 75-77o/3mm). It sublimes in a very high vacuum. The acetate crystallises from aqueous MeOH (m 178o, 180o) or EtOH or AcOH (m 173-174o) and has b 331.3o/760mm. [Beilstein 12 III 1325, 12 IV 1116.]
Properties of 4-Chloroaniline
| Melting point: | 67-70 °C (lit.) |
| Boiling point: | 232 °C (lit.) |
| Density | 1,43 g/cm3 |
| vapor density | 4.4 (vs air) |
| vapor pressure | 0.15 mm Hg ( 25 °C) |
| refractive index | 1.5546 |
| Flash point: | 120°C |
| storage temp. | Store below +30°C. |
| solubility | 2.2g/l |
| form | Crystalline Solid |
| pka | 4.15(at 25℃) |
| color | Beige to brown-purple |
| Specific Gravity | 1.2417 |
| PH Range | 6.9 at 1.00000 g/l at 20 °C |
| Odor | Sweetish |
| PH | 6.9 (1g/l, H2O, 20℃) |
| Water Solubility | 0.3 g/100 mL (20 ºC) |
| Merck | 14,2118 |
| BRN | 471359 |
| Henry's Law Constant | 1.07 at 25 °C (calculated, Howard, 1989)(x 10-5 atm?m3/mol) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides, chloroformates, nitrous acid. May be light sensitive. |
| CAS DataBase Reference | 106-47-8(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Chloroaniline(106-47-8) |
| IARC | 2B (Vol. 57) 1993 |
| EPA Substance Registry System | p-Chloroaniline (106-47-8) |
Safety information for 4-Chloroaniline
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 4-Chloroaniline
4-Chloroaniline manufacturer
JSK Chemicals
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



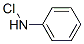
You may like
-
 4-Chloroaniline 98%View Details
4-Chloroaniline 98%View Details -
 4-Chloroaniline 98%View Details
4-Chloroaniline 98%View Details -
 4-Chloroaniline 99%View Details
4-Chloroaniline 99%View Details -
 4-Chloroaniline, 98% CAS 106-47-8View Details
4-Chloroaniline, 98% CAS 106-47-8View Details
106-47-8 -
 p-Chloroaniline, GR 99%+ CAS 106-47-8View Details
p-Chloroaniline, GR 99%+ CAS 106-47-8View Details
106-47-8 -
 p-Chloroaniline CAS 106-47-8View Details
p-Chloroaniline CAS 106-47-8View Details
106-47-8 -
 4-Chloroaniline CAS 106-47-8View Details
4-Chloroaniline CAS 106-47-8View Details
106-47-8 -
 4-Chloroaniline CAS 106-47-8View Details
4-Chloroaniline CAS 106-47-8View Details
106-47-8
