4-Bromoaniline
Synonym(s):4-Bromoaniline;p-Bromoaniline, p-Bromophenylamine
- CAS NO.:106-40-1
- Empirical Formula: C6H6BrN
- Molecular Weight: 172.02
- MDL number: MFCD00007822
- EINECS: 203-393-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
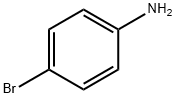
What is 4-Bromoaniline?
Chemical properties
Gray-brown crystals with a peculiar odor. When heated or burned, they break down and release harmful fumes that contain hydrogen bromide and nitrogen oxides. When dissolved in water, it acts as a mild base. It reacts with acids and powerful oxidizing agents. The compound 4-bromoaniline consists of a hydrophobic benzene ring and an amino group that assists in carrying substances and improving PSC stability.
The Uses of 4-Bromoaniline
4-Bromoaniline is a brominated aniline used as a building block in the preparation or pharmaceutial and organic compounds.
What are the applications of Application
4-Bromoaniline is a brominated aniline used as a building block
Preparation
4-bromoaniline can be made by reacting aniline with bromine with a protection with acetyl chloride.
Synthesis Reference(s)
The Journal of Organic Chemistry, 40, p. 1867, 1975 DOI: 10.1021/jo00900a053
Synthetic Communications, 19, p. 3047, 1989 DOI: 10.1080/00397918908052699
General Description
P-bromoaniline is a brown solid with a sweet odor. (NTP, 1992)
Air & Water Reactions
4-Bromoaniline is sensitive to prolonged exposure to air. Vapor may form highly reactive mixtures in air . Insoluble in water.
Reactivity Profile
Vapor may form highly reactive mixtures in air.
Fire Hazard
Flash point data are not available for 4-Bromoaniline, but 4-Bromoaniline is probably combustible.
Synthesis
RGO/Cu NPs (25 mg), arylboronic acid (1.0 mmol), K2CO3(1.3 mmol), 25-28% aqueous ammonia (5 mmol) and methanol(4 mL) were added to a 50 mL round-bottomed flask. The reactionmixture was stirred under reflux conditions for the appropriatetime. After completion of the reaction as monitored by TLC, themixture was filtered, and the solvent of the filtrate was removedunder vacuum with the aid of a rotary evaporator. The residue waspurified by column chromatography on silica gel to afford 4-Bromoaniline.
Purification Methods
Crystallise the aniline (with appreciable loss) from aqueous EtOH. The benzoyl derivative has m 204o (from EtOH). [Beilstein 12 IV 1497.]
Properties of 4-Bromoaniline
| Melting point: | 56-62 °C (lit.) |
| Boiling point: | 230-250 °C |
| Density | 1.497 |
| refractive index | 1.5680 (estimate) |
| Flash point: | 222-224°C |
| storage temp. | Store below +30°C. |
| solubility | ethanol: soluble0.5g/10 mL, clear, colorless to almost colorless |
| form | crystalline |
| pka | 3.86(at 25℃) |
| color | white to light yellow |
| Odor | Sweetish |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 14,1404 |
| BRN | 742031 |
| Dielectric constant | 13.0(-7℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, peroxides, acids, acid chlorides, acid anhydrides, chloroformates. May be air sensitive. |
| CAS DataBase Reference | 106-40-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Bromoaniline(106-40-1) |
| EPA Substance Registry System | p-Bromoaniline (106-40-1) |
Safety information for 4-Bromoaniline
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for 4-Bromoaniline
| InChIKey | WDFQBORIUYODSI-UHFFFAOYSA-N |
4-Bromoaniline manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4-Bromoaniline 106-40-1 99%View Details
4-Bromoaniline 106-40-1 99%View Details
106-40-1 -
 106-40-1 4-Bromo aniline 98%View Details
106-40-1 4-Bromo aniline 98%View Details
106-40-1 -
 4-Bromoaniline 106-40-1 98%View Details
4-Bromoaniline 106-40-1 98%View Details
106-40-1 -
 4-Bromoaniline CAS 106-40-1View Details
4-Bromoaniline CAS 106-40-1View Details
106-40-1 -
 4-Bromoaniline CAS 106-40-1View Details
4-Bromoaniline CAS 106-40-1View Details
106-40-1 -
 three Bromoaniline chemicalView Details
three Bromoaniline chemicalView Details
106-40-1 -
 4-Bromoaniline, 99%, 25/ 50 kgView Details
4-Bromoaniline, 99%, 25/ 50 kgView Details
106-40-1 -
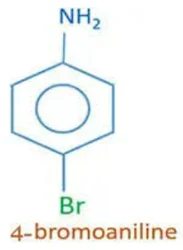 4- Bromo aniline CAS no. 106-40-1, 98%View Details
4- Bromo aniline CAS no. 106-40-1, 98%View Details
106-40-1
