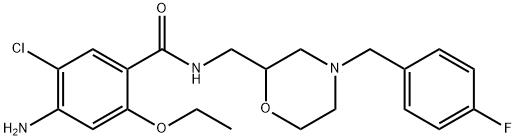
4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
- CAS NO.:90182-92-6
- Empirical Formula: C15H20ClN3O2
- Molecular Weight: 309.79
- MDL number: MFCD04971977
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
![4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE Structural](https://img.chemicalbook.in/CAS/GIF/90182-92-6.gif)
What is 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE?
Originator
Zacopride hydrochloride,ZYF Pharm Chemical
The Uses of 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
Anti-emetic; stimulant (peristaltic).
The Uses of 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
Zacopride is a highly potent SR-3 antagonist and SR-4 agonist. 5-HT3 receptor antagonist. Activates IK1 channel.
Definition
ChEBI: 4-amino-N-(1-azabicyclo[2.2.2]octan-3-yl)-5-chloro-2-methoxybenzamide is a member of benzamides.
Manufacturing Process
4-Amino-N-(1-azabicyclo[2.2.2]oct-3-yl)-5-chloro-2-methoxybenzamide,
fumarate [1:1]:
In a closed system equipped with an oil bubbler, 30 ml of tetrahydrofuran
were added to a mixture of 4-amino-5-chloro-2-methoxybenzoic acid, 2.02 g
(0.010 mole) and 1,1'-carbonyldiimidazole, 1.62 g (0.010 mole) with stirring.
When evolution of carbon dioxide ceased, nitrogen was bubbled through the
reaction mixture for 1 hr. A solution of 3-aminoquinuclidine, 1.26 g (0.010
mole) in 10 ml tetrahydrofuran was added dropwise to the stirred reaction
mixture and stirring at room temperature continued for 3 hrs. TLC analysis
(3% conc. ammonium hydroxide solution in methanol) showed some product
formation. The mixture was heated at reflux temperature for 18 hours and
then concentraded to an oil. TLC analysis showed the presence of the product,
imidazole and 3-aminoquinuclidine. The oil was dissolved in methylene
chloride (75 ml) and washed twice with 50 ml portions of aqueous sodium
bicarbonate solution. The methylene chloride layer was dried over anhydrous
magnesium sulfate and concentrated to yield 2.0 g (67%) of a glassy
amorphous solid, the free base of the title compound.
In another reaction on a 0.020 mole scale, 5.18 g (83.8%) of the product as
the free base was obtained.
The products were combined, dissolved in methanol (20 ml) and the solution
and treated with a solution of fumaric acid (2.73 g) in methanol (50 ml).
Absolute ether was added to precipitate the salt which was collected by
filtration and recrystallized from methanol-water (200:20) with isopropyl ether
added to the point of incipient cloudiness. The recrystallized salt (5.38 g)
melted at 223°-225°C.
4-Amino-N-(1-azabicyclo[2.2.2]oct-3-yl)-5-chloro-2-methoxybenzamide,
hydrochloride, hydrate (1:1:1):
To an isopropyl alcohol solution of the above free base of the title compound
is added in equal molar amount of 37% (conc.) hydrochloric acid. A salt is separated by addition of acetone followed by filtration which is recrystallized
from acetone-water to give the title compound, MP: 158°-160°C.
Therapeutic Function
Antiemetic, Peristaltic stimulant
Biological Activity
Highly potent 5-HT 3 receptor antagonist (K i = 0.38 nM) and 5-HT 4 receptor agonist (K i = 373 nM). Antiemetic and anxiolytic following systemic administration in vivo .
Properties of 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
| storage temp. | Desiccate at RT |
Safety information for 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
Computed Descriptors for 4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-AMINO-N-1-AZABICYCLO[2.2.2]OCT-3-YL-5-CHLORO-2-METHOXYBENZAMIDE HYDROCHLORIDE](https://img.chemicalbook.in/CAS/GIF/90182-92-6.gif)
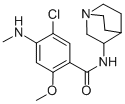
![Zacopride hydrochloride [USAN:INN]](https://img.chemicalbook.in/CAS/20211123/GIF/99617-34-2.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1