4-Acetoxyindole
Synonym(s):4-Acetoxyindole
- CAS NO.:5585-96-6
- Empirical Formula: C10H9NO2
- Molecular Weight: 175.18
- MDL number: MFCD00010678
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-18 13:26:42
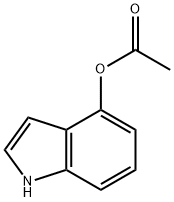
What is 4-Acetoxyindole?
Description
4-acetoxylindole is also known as 4-Indolyl acetate. It can be used as standards of the reverse phase HPLC during separation of small molecules. It can also be used as reactant during the preparation of inhibitors of HIV-attachment, prostanoid EP3 antagonist, indolyl-nitroalkanes and psilocybin.
The Uses of 4-Acetoxyindole
4-Acetoxyindole is a useful intermediate for organic synthesis of Psilocine and Psilocine analogs.
The Uses of 4-Acetoxyindole
Reactant for preparation of:• ;Inhibitors of HIV-1 attachment1• ;Prostanoid EP3 receptor antagonist, as a novel antiplatelet agent2• ;Indolyl-nitroalkanes via N-bromosuccinimide catalyzed synthesis3• ;Psilocybin as a medicinal agent4
The Uses of 4-Acetoxyindole
Reactant for preparation of:
- Inhibitors of HIV-1 attachment
- Prostanoid EP3 receptor antagonist, as a novel antiplatelet agent
- Indolyl-nitroalkanes via N-bromosuccinimide catalyzed synthesis
- Psilocybin as a medicinal agent
General Description
4-Acetoxyindole is a chromophore that belongs to the pyrrole family of compounds. It has been shown to react with an ionic liquid under acidic conditions to form a protonated intermediate, which can be deprotonated by a nucleophile. This reaction yields an acetate anion and a fluorescing product. 4-Acetoxyindole also reacts with deuterium gas, yielding an acetate, a deuterium atom, and fluorescing product. The reaction is reversible and the yield of the product depends on the concentration of the reactants. 4-Acetoxyindole has strong carbonyl groups that make it reactive towards other functional groups. These reactions are useful for synthesizing heterocycles such as indoles and isoquinolines.
References
http://www.sigmaaldrich.com/catalog/product/aldrich/259047?lang=en®ion=US
https://pubchem.ncbi.nlm.nih.gov/compound/4-Acetoxyindole
Properties of 4-Acetoxyindole
| Melting point: | 100-102°C |
| Boiling point: | 339.1±15.0 °C(Predicted) |
| Density | 1?+-.0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 16.38±0.30(Predicted) |
| color | Off-White to Beige |
| InChI | InChI=1S/C10H9NO2/c1-7(12)13-10-4-2-3-9-8(10)5-6-11-9/h2-6,11H,1H3 |
| CAS DataBase Reference | 5585-96-6(CAS DataBase Reference) |
Safety information for 4-Acetoxyindole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Acetoxyindole
| InChIKey | ZXDMUHFTJWEDEF-UHFFFAOYSA-N |
| SMILES | N1C2=C(C(OC(=O)C)=CC=C2)C=C1 |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 5585-96-6 4-Indoxyl acetate 98%View Details
5585-96-6 4-Indoxyl acetate 98%View Details
5585-96-6 -
 4-Indolyl acetate CAS 5585-96-6View Details
4-Indolyl acetate CAS 5585-96-6View Details
5585-96-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4