4-Acetamidobenzenesulfonyl azide
Synonym(s):p-ABSA
- CAS NO.:2158-14-7
- Empirical Formula: C8H8N4O3S
- Molecular Weight: 240.24
- MDL number: MFCD00029626
- EINECS: 606-801-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
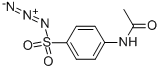
What is 4-Acetamidobenzenesulfonyl azide?
The Uses of 4-Acetamidobenzenesulfonyl azide
4-Acetamidobenzenesulfonyl azide is used as a hydroazidation catalyst for facile preparation of organoazides. It is used as a reagent for synthesis of: monosaccharide-derived alcohols, a late-stage intermolecular C-H olefination, intramolecular isomuenchnone cycloaddition approach to antitumor agents, rhodium-catalyzed carbene cyclization cycloaddition cascade reaction of vinylsulfonates and Suzuki-Miyaura cross coupling reaction.
The Uses of 4-Acetamidobenzenesulfonyl azide
Hydroazidation Catalyst for Facile Preparation of Organoazides
Diazo transfer agent.
Synthesis
To a solution of CCA(0.1548 g, 0.6 mmol) in tetrahydrofuran (3-5 mL), PPh3 (0.5246 g, 2 mmol)was added at 0-5°C with stirring. A white suspension was formed to which p-toluenesulfonic acid (0.1720 g, 1 mmol) was added and stirring continued for 15 min. NaN3 (0.065 g, 1 mmol) was added and the temperature was raised up to room temperature. Stirring was continued for 1 min at room temperature. After completion of the reaction (TLC), the reaction mixture was concentrated, washed with EtOAc (4-6 mL), and cold distilled water (5 mL). The organic layer was dried with anhydrous Na2SO4, passed through a short silica-gel column using n-hexane/ethylacetate (10/1) as eluent. 4-Acetamidobenzenesulfonyl azide was obtained after removing the solvent under reduced pressure.
structure and hydrogen bonding
Under ambient conditions, 4-acetamidobenzenesulfonyl azide (4-ABSA) belongs to a monoclinic structure with a P21 space group and cell parameters of a = 8.0529 Å, b = 22.988 Å, c = 8.3123 Å, β=93.534°, respectively. The hydrogen-bonding interactions allow molecules to pair up and form π-stacked dimers. The studies of NH4N3 have reported that the hydrogen bonds are affected by the rotation of azide ions with increasing pressure. The cooperativity of hydrogen bonds and π-stacking interactions allow 4-ABSA to become an attractive candidate for studying the influence of non-covalent interactions within the organic azides in the compression process. In addition, the bent azide groups of 4-ABSA are crucial for electron orbit hybridization and nitrogen polymerization.
References
[1] Junru Jiang. “High-Pressure Studies of 4-Acetamidobenzenesulfonyl Azide: Combined Raman Scattering, IR Absorption, and Synchrotron X-ray Diffraction Measurements.” The Journal of Physical Chemistry B 120 46 (2016): 12015–12022.
Properties of 4-Acetamidobenzenesulfonyl azide
| Melting point: | 107-111 °C (lit.) |
| storage temp. | 2-8°C |
| form | powder to crystal |
| color | White to Amber to Dark purple |
| Water Solubility | Insoluble in water. |
| BRN | 2219568 |
| InChI | InChI=1S/C8H8N4O3S/c1-6(13)10-7-2-4-8(5-3-7)16(14,15)12-11-9/h2-5H,1H3,(H,10,13) |
| CAS DataBase Reference | 2158-14-7(CAS DataBase Reference) |
Safety information for 4-Acetamidobenzenesulfonyl azide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Acetamidobenzenesulfonyl azide
| InChIKey | NTMHWRHEGDRTPD-UHFFFAOYSA-N |
| SMILES | C1(S(=O)(=O)N=[N+]=[N-])=CC=C(NC(=O)C)C=C1 |
4-Acetamidobenzenesulfonyl azide manufacturer
SAKEM LLP
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 2158-14-7 4-ACETAMIDO BENZENE SULPHONYL AZIDE 98%View Details
2158-14-7 4-ACETAMIDO BENZENE SULPHONYL AZIDE 98%View Details
2158-14-7 -
 4-Acetamidobenzenesulfonyl azide 2158-14-7 98%View Details
4-Acetamidobenzenesulfonyl azide 2158-14-7 98%View Details
2158-14-7 -
 4-Acetamidobenzenesulfonyl azide CAS 2158-14-7View Details
4-Acetamidobenzenesulfonyl azide CAS 2158-14-7View Details
2158-14-7 -
 4-Acetamidobenzenesulfonyl Azide CAS 2158-14-7View Details
4-Acetamidobenzenesulfonyl Azide CAS 2158-14-7View Details
2158-14-7 -
 4-Acetamidobenzenesulfonyl azide CAS 2158-14-7View Details
4-Acetamidobenzenesulfonyl azide CAS 2158-14-7View Details
2158-14-7 -
 4-Acetamido Benzene Sulphonyl Azide-Bulk 98%View Details
4-Acetamido Benzene Sulphonyl Azide-Bulk 98%View Details
2158-14-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
