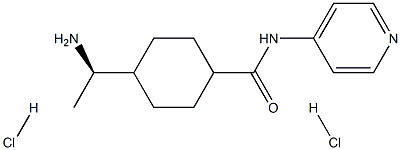
4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
Synonym(s):4-[6-(4-Isopropoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl]quinoline;4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline;ALK2/3 Inhibitor, DMH1, Bone Morphogenetic Protein Inhibitor, DMH1, 4-(6-(4-Isopropoxyphenyl)pyrazolo[1,5-a]pyrimidin-3-yl)quinoline;BMP Inhibitor II, DMH1 - CAS 1206711-16-1 - Calbiochem
- CAS NO.:1206711-16-1
- Empirical Formula: C24H20N4O
- Molecular Weight: 380.44
- MDL number: MFCD18384963
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
![4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline Structural](https://img.chemicalbook.in/CAS/GIF/1206711-16-1.gif)
What is 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline?
Description
Bone morphogenetic proteins (BMP) are secreted signaling proteins, many of which are involved in various developmental processes, in addition to bone formation. DMH1 is an analog of the non-selective BMP receptor inhibitor dorsomorphin that potently inhibits the kinase activity of activin receptor-like kinase 2 (ALK2; IC50 = 13-108 nM). It is much less effective at ALK4, ALK5, AMPK, KDR (VEGFR2) or PDGFRβ, although it inhibits ALK1 and ALK3 at nanomolar concentrations. DMH1 is effective in vivo, as it disrupts dorsoventral development in zebrafish. It also affects stem cell development, increasing cardiomyocyte progenitors and promoting neurogenesis. DMH1 inhibits the growth of lung cancer cells, reducing tumor growth in a xenograft mouse model.
The Uses of 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
DMH 1 is an inhibitor that exclusively targets the bone morphogenetic protein (BMP) but not the vascular endothelial growth factor (VEGF) pathway. DMH 1 promotes neurogenesis of human-induced pluripotent stem cells (hiPSCs)
The Uses of 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
DMH1 has been used for bone morphogenetic protein (BMP) inhibition. It has also been used to block vascular endothelial growth factor (VEGF) signaling.
What are the applications of Application
DMH-1 is a potent, selective inhibitor of the bone morphogenic protein (BMP) ALK2 receptor, analogous to the BMP inhibitor dorsomorphin.
Definition
ChEBI: DMH1 is a pyrazolopyrimidine that is pyrazolo[1,5-a]pyrimidine bearing quinolin-4-yl and 4-isopropyloxyphenyl substituents at positions 3 and 6 respectively. It has a role as a protein kinase inhibitor, a bone morphogenetic protein receptor antagonist and an antineoplastic agent. It is a member of quinolines, a pyrazolopyrimidine and an aromatic ether.
General Description
DMH1 stimulates neurogenesis of human-induced pluripotent stem cells (hiPSCs) and suppresses the growth and metastasis of lung cancer. It blocks cellular autophagy responses.
Biochem/physiol Actions
DMH1 is a highly selective Bone morphogenetic protein (BMP) inhibitor. DMH1 is a dorsomorphin analogue that exclusively targets the BMP but not the VEGF pathway.
storage
+4°C
References
[1] hao j, ho j n, lewis j a, et al. in vivo structure- activity relationship study of dorsomorphin analogues identifies selective vegf and bmp inhibitors. acs chemical biology, 2010, 5(2): 245-253.
[2] hao j, lee r, chang a, et al. dmh1, a small molecule inhibitor of bmp type i receptors, suppresses growth and invasion of lung cancer. plos one, 2014, 9(3): e90748.
Properties of 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
| Melting point: | 178 °C |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| pka | 3.34±0.46(Predicted) |
| color | light yellow to orange |
Safety information for 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-[6-[4-(1-Methylethoxy)phenyl]pyrazolo[1,5-a]pyrimidin-3-yl]-quinoline
New Products
3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Iodophenylacetic acid Cyclohexane, (2-propynyloxy)- (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine Pivalic anhydride,98% Phenylmethanesulfonyl fluoride, 98% Glyoxylic acid solution, 50% in water tert-Butyl glycinate,97% 4-Ethoxybenzoic acid, 99% Sodium 1-octanesulfonate monohydrate 7-Ethyl Tryptophol 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 3-Methoxybenzonitrile Dibenzoyl Peroxide 4-Methoxybenzonitrile Titanium Dioxide Chloral PentachlorobenzonitrileRelated products of tetrahydrofuran
![(2S)-1-[3-Ethyl-7-[[(1-oxido-3-pyridinyl)methyl]amino]pyrazolo[1,5-a]pyrimidin-5-yl]-2-piperidineethanol](https://img.chemicalbook.in/CAS/GIF/779353-01-4.gif)
You may like
-
 DMH1 >95% CAS 1206711-16-1View Details
DMH1 >95% CAS 1206711-16-1View Details
1206711-16-1 -
 DMH-1 CAS 1206711-16-1View Details
DMH-1 CAS 1206711-16-1View Details
1206711-16-1 -
 DMH1 CAS 1206711-16-1View Details
DMH1 CAS 1206711-16-1View Details
1206711-16-1 -
 BMP Inhibitor II, DMH1 CAS 1206711-16-1View Details
BMP Inhibitor II, DMH1 CAS 1206711-16-1View Details
1206711-16-1 -
 Avibactam seriesView Details
Avibactam seriesView Details
1192651-80-1 -
 Avibactam Sodium SaltView Details
Avibactam Sodium SaltView Details
1192491-61-4 -
 Avibactam intermediateView Details
Avibactam intermediateView Details
1171080-45-7 -
 Relibatan intermediateView Details
Relibatan intermediateView Details
1416134-63-8
