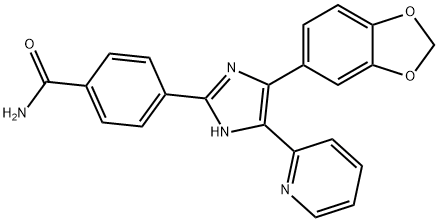
SB 431542
Synonym(s):4-(5-Benzol[1,3]dioxol-5-yl-4-pyrldin-2-yl-1H-imidazol-2-yl)-benzamide hydrate;4-[4-(1,3-Benzodioxol-5-yl)-5-(2-pyridinyl)-1H-imidazol-2-yl]-benzamide hydrate;4-[4-(3,4-Methylenedioxyphenyl)-5-(2-pyridyl)-1H-imidazol-2-yl]-benzamide hydrate;
- CAS NO.:301836-41-9
- Empirical Formula: C22H16N4O3
- Molecular Weight: 384.39
- MDL number: MFCD05865244
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
What is SB 431542?
Description
SB-431542 is a potent and selective inhibitor of the TGF-
The Uses of SB 431542
SB 431542 is a known Src family kinase inhibitor that effectively blocks the transforming growth factor-β1-induced cell migration and invasion in both established and primary carcinoma cells.
The Uses of SB 431542
SB 431542 is a known Src family kinase inhibitor that effectively blocks the transforming growth factor-β1-induced cell migration and invasion in both established and primary carcinoma cells. Potent TGF-beta inhibitor.
What are the applications of Application
SB 431542 is a Potent, selective inhibitor of TGF-βRI, specifically at ALK5 and its relatives ALK4 and ALK7.
Definition
ChEBI: A member of the class of benzamides that is 4-(imidazol-2-yl)benzamide carrying additional 1,3-benzodioxol-5-yl and pyridin-2-yl substituents at positions 4 and 5 respectively on the imidazole ring.
General Description
A cell-permeable triarylimidazole compound that is shown to effectively inhibit cellular Smad2 phosphorylation (>90% inhibition by 10 μM inhibitor) upon vector-mediated expression of constitutively active ALK4, ALK5, or ALK7 in NIH 3T3 cells, while exhibiting little effect against Smad1 phosphorylation by other members of type I receptors for TGF-β in NIH 3T3 cultures expressing active ALK1, 2, 3, or 6. When tested directly in cell-free kinase assays, SB431542 is demonstrated to potently inhibit the activity of ALK4 and ALK5 (IC50 = 140 nM and 94 nM, respectively) with no or much reduced potency toward a panel of 24 other kinases (IC50 ≥10 μM in the presence of 10 μM ATP), including ALK2 and ALK6. Reported to improve the efficiency of 4-TF-induced human iPSCs generation from fibroblast cultures by >200-fold when used together with PD0325901 (Cat. No. 444966) and Thiazovivin (Cat. No. 420220).
Biological Activity
Potent and selective inhibitor of the transforming growth factor- β (TGF- β ) type I receptor activin receptor-like kinase ALK5 (IC 50 = 94 nM), and its relatives ALK4 and ALK7. Suppresses TGF- β -induced proliferation of human osteosarcoma cells. Stimulates proliferation, differentiation and sheet formation of ESC-derived endothelial cells.
Biochem/physiol Actions
SB-431542 inhibits the TGF-β-mediated activation of SMAD proteins, expression of collagen and fibronectin, cell proliferation and cell motility. It does not inhibit kinases that are activated in response to serum or stress such as ERK, p38 or JNK.
storage
Room temperature
References
1) Laping et al. (2002), Inhibition of transforming growth factor (TGF-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I kinase activity: SB-431542; Mol. Pharmacol., 62 58 2) Inman et al. (2002), SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5 and ALK7; Mol. Pharmacol., 62 65 3) Matsuyama et al. (2003), SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells; Cancer Res., 63 7791 4) Watabe et al. (2003), TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells; J. Cell Biol., 163 1303 5) Stanslowsky et al. (2014), Functional differentiation of midbrain neurons from human cord blood-derived induced pluripotent stem cells; Stem Cell Res. Ther., 5 35
Properties of SB 431542
| Melting point: | 214 °C(dec.) |
| Boiling point: | 662.4±55.0 °C(Predicted) |
| Density | 1.373 |
| Flash point: | 354.4℃ |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO: 10 mg/mL, soluble |
| form | powder |
| pka | 10.14±0.10(Predicted) |
| color | Yellow |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for SB 431542
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SB 431542
| InChIKey | FHYUGAJXYORMHI-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![6-[2-tert-Butyl-5-(6-methyl-pyridin-2-yl)-1H-imidazol-4-yl]-quinoxaline](https://img.chemicalbook.in/CAS/GIF/356559-20-1.gif)
![2-(5-BENZO[1,3]DIOXOL-5-YL-2-TERT-BUTYL-3H-IMIDAZOL-4-YL)-6-METHYLPYRIDINE HYDROCHLORIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure9/GIF/CB6719733.gif)
You may like
-
 SB-431542 >95% CAS 301836-41-9View Details
SB-431542 >95% CAS 301836-41-9View Details
301836-41-9 -
 SB 431542 99% (HPLC) CAS 301836-41-9View Details
SB 431542 99% (HPLC) CAS 301836-41-9View Details
301836-41-9 -
 SB 431542 CAS 301836-41-9View Details
SB 431542 CAS 301836-41-9View Details
301836-41-9 -
 SB 431542 hydrate ≥98% (HPLC), powder CAS 301836-41-9View Details
SB 431542 hydrate ≥98% (HPLC), powder CAS 301836-41-9View Details
301836-41-9 -
 TGF-β RI Kinase Inhibitor VI, SB431542 CAS 301836-41-9View Details
TGF-β RI Kinase Inhibitor VI, SB431542 CAS 301836-41-9View Details
301836-41-9 -
 SB 431542 hydrate CAS 301836-41-9View Details
SB 431542 hydrate CAS 301836-41-9View Details
301836-41-9 -
 TGF-β RI Kinase Inhibitor VI, SB431542 CAS 301836-41-9View Details
TGF-β RI Kinase Inhibitor VI, SB431542 CAS 301836-41-9View Details
301836-41-9 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1