Hydroxytyrosol
Synonym(s):2-(3,4-Dihydroxyphenyl)ethanol;3,4-Dihydroxyphenethyl alcohol;DOPET;Homoprotocatechuyl alcohol
- CAS NO.:10597-60-1
- Empirical Formula: C8H10O3
- Molecular Weight: 154.16
- MDL number: MFCD01320529
- EINECS: 600-704-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
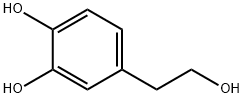
What is Hydroxytyrosol ?
Description
3,4-Dihydroxyphenyl ethanol is a phenolic component of olive oil that inhibits both 12- and 5-LO. The IC50 values for the inhibition of rat platelet 12-LO and rat neutrophil 5-LO are 4.2 and 13 μM, respectively. It does not inhibit, and may actually enhance, COX activity. 3,4-Dihydroxyphenyl ethanol also protects LDL from both biological and chemical oxidation, suggesting a potential mechanism for the protective effects of olive oil against atherosclerosis.
Chemical properties
Brown oil is derived from Olea europaea leaves and is soluble in methanol, DMSO, and other solvents but insoluble in petroleum ether, ether, and chloroform. The molecular structure of hydroxytyrosol is created by replacing the hydrogen atom on the methyl group of ethanol with catechol. It possesses an alcohol hydroxyl group in the chain of ethanol with a benzene ring, along with phenolic hydroxyl groups like other phenolic substances. Its antioxidant activity is closely linked to its structure.
Characteristics
Hydroxytyrosol displays much more effective antioxidant characteristics, such as the scavenging of free radicals, breaking peroxidative chain reactions, preventing lipid peroxidation, inhibiting hypochlorous acid derived radicals, and so on, compared with other phenolic compounds in olive oil. It could be used in the dermocosmetic industry for the creation of products for protecting the skin from oxidative stress or used as a preservative in the food technology.
The Uses of Hydroxytyrosol
Hydroxytyrosol is used in cardiovascular drugs, and has miraculous effects on the prevention and treatment of arteriosclerosis, hypertension, heart disease, cerebral hemorrhage, etc., and is superior to similar drugs. It can also Inhibits the proliferation rate of cancer cells and induces apoptosis of cancer cells.
The Uses of Hydroxytyrosol
Hydroxy tyrosol is a tyrosol metabolite. It is also a strong anti-oxidant found in olive oil. It is used in beauty products and health care products, which can effectively enhance skin elasticity and moisturizing, and has the effect of anti-wrinkle and anti-aging.
What are the applications of Application
3,4-Dihydroxyphenyl Ethanol is a phenolic antioxidant inhibitor of 5-LO (5-lipoxygenase) and 12-LO (12-lipoxygenase)
Definition
ChEBI: Hydroxytyrosol is a member of the class of catechols that is benzene-1,2-diol substituted by a 2-hydroxyethyl group at position 4. Isolated from Olea europaea, it exhibits antioxidant and antineoplastic activities. It has a role as a metabolite, an antioxidant and an antineoplastic agent. It is a member of catechols and a primary alcohol. It derives from a 2-(4-hydroxyphenyl)ethanol.
General Description
Hydroxytyrosol, found in olives, is a potent antioxidant in our bodies. It is considered one of the most powerful antioxidants, with an oxygen radical absorption capacity of about 4,500,000 μmolTE/100g. This is 10 times greater than green tea and more than double the antioxidant capacity of CoQ10 and oak extract. Hydroxytyrosol is used in cardiovascular drugs, and has miraculous effects on the prevention and treatment of arteriosclerosis, hypertension, heart disease, cerebral hemorrhage, etc., and is superior to similar drugs. It can also Inhibits the proliferation rate of cancer cells and induces apoptosis of cancer cells.
Biological Activity
Hydroxytyrosol is a phenolic component of olive oil that inhibits both 12- and 5-LO. The IC50 values for the inhibition of rat platelet 12-LO and rat neutrophil 5-LO are 4.2 and 13 μM, respectively. It does not inhibit, and may actually enhance, COX activity. Hydroxytyrosol also protects LDL from both biological and chemical oxidation, suggesting a potential mechanism for the protective effects of olive oil against atherosclerosis.
Biochem/physiol Actions
Metabolite of oleuropein. Antioxidant. Inhibits the rate of cancer cell proliferation and induces cancer cell apoptosis.
Mechanism of action
Hydroxytyrosol (3,4-dihydroxyphenylethanol, HTyr), a phenolic alcohol, has been hypothesized to exert a wide range of biological effects, cardioprotective, anticancer, neuroprotective antimicrobial, beneficial endocrine, and other effects. Initially, the wide variety of HTyr biological activities was associated with its potent antioxidant activity. HTyr acts as a free radical scavenger and metal chelator. The high antioxidant efficiency of HTyr is attributed to the presence of the o-dihydroxyphenyl moiety. It mainly acts as a chain breaker by donating a hydrogen atom to peroxyl-radicals (ROO*). In this way, fairly reactive ROO* is replaced with HTyr* radical, unreactive due to intramolecular hydrogen bonds in the phenoxy radical. However, it has been proposed that HTyr may confer additional antioxidant protection by increasing the endogenous defense systems against oxidative stress by activating different cellular signaling pathways. One proposed mechanism involves the HTyr-mediated induction of phase II detoxifying enzymes via nuclear factor E2-related factor 2 (Nrf2) activation in different tissues.
Side Effects
Hydroxytyrosol (HTyr) is a phenolic alcohol considered by the nutraceutical and food industries to be an excellent food supplement with no known adverse effects.The no observed adverse effect level for HTyr is 50 mg/kg body weight per day. The EFSA Panel on Nutritional Products, Nutrition and Allergies (NDA) on the safety of hydroxytyrosol as a novel food concluded that the MoE at the intended use and use level is adequate for the target population. The Panel concluded that the novel food hydroxytyrosol was safe at the proposed use and use levels.
Source
Hydroxytyrosol, also known as 3,4-Dihydroxyphenylethanol(3,4-DHPEA) or DOPET, is primarily found in olive oil as secoiridoid derivatives and in its acetate and free forms. Both hydroxytyrosol and its derivatives arise from oleuropein (hydroxytyrosol esterified with elenolic acid), present in olives during the extraction of olive oil.
Wine has proven to be another important source of hydroxytyrosol in the Mediterranean diet, and is formed in wine from tyrosol during alcoholic fermentation. Hydroxytyrosol was firstly found in Italian wines by Di Tommaso et al., and later in other Italian and Greek wines. Some authors describe a higher concentration in red wines (3.66-4.20 mg/L-1) than in white wines (1.72-1.92mg/L-1). Finally, Minuti et al. obtained hydroxytyrosol concen- trations between 1.8 and 3.1 mg L-1 in red wine. Thus, scientific literature shows that wine is an important source of hydroxytyrosol in the diet, along with olive oil.
Properties of Hydroxytyrosol
| Boiling point: | 355.4±27.0 °C(Predicted) |
| Density | 1.321±0.06 g/cm3(Predicted) |
| refractive index | 1.5810-1.5860 |
| storage temp. | -20°C |
| solubility | Acetonitrile (Slightly), DMSO (Slightly), Ethyl Acetate (Sparingly), Methanol (Slightly) |
| form | Solid |
| pka | 9.72±0.10(Predicted) |
| color | Colourless to Yellow Sticky Oil |
| BRN | 2208118 |
| Stability: | Hygroscopic |
| InChI | InChI=1S/C8H10O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,9-11H,3-4H2 |
| CAS DataBase Reference | 10597-60-1(CAS DataBase Reference) |
Safety information for Hydroxytyrosol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Hydroxytyrosol
| InChIKey | JUUBCHWRXWPFFH-UHFFFAOYSA-N |
| SMILES | C1(O)=CC=C(CCO)C=C1O |
Hydroxytyrosol manufacturer
EMBIO LIMITED
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 3,4-Dihydroxyphenylethanol 99%View Details
3,4-Dihydroxyphenylethanol 99%View Details -
 10597-60-1 99%View Details
10597-60-1 99%View Details
10597-60-1 -
 3,4-Dihydroxyphenylethanol 98%View Details
3,4-Dihydroxyphenylethanol 98%View Details
10597-60-1 -
 3-Hydroxytyrosol CAS 10597-60-1View Details
3-Hydroxytyrosol CAS 10597-60-1View Details
10597-60-1 -
 3-Hydroxytyrosol, 98% CAS 10597-60-1View Details
3-Hydroxytyrosol, 98% CAS 10597-60-1View Details
10597-60-1 -
 3-Hydroxytyrosol CAS 10597-60-1View Details
3-Hydroxytyrosol CAS 10597-60-1View Details
10597-60-1 -
 Hydroxytyrosol CAS 10597-60-1View Details
Hydroxytyrosol CAS 10597-60-1View Details
10597-60-1 -
 3-Hydroxytyrosol CAS 10597-60-1View Details
3-Hydroxytyrosol CAS 10597-60-1View Details
10597-60-1
