3-Pyridinemethanol
Synonym(s):ω-Hydroxy-3-picoline;3-(Hydroxymethyl)pyridine;3-Pyridyl carbinol
- CAS NO.:100-55-0
- Empirical Formula: C6H7NO
- Molecular Weight: 109.13
- MDL number: MFCD00006407
- EINECS: 202-864-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08

What is 3-Pyridinemethanol?
Chemical properties
CLEAR LIGHT YELLOW TO YELLOW LIQUID
Originator
Roniacol,Roche,US,1949
The Uses of 3-Pyridinemethanol
3-Pyridinemethanol can be used as antineoplastic and used in the synthesis of histone deacetylase inhibitors.
The Uses of 3-Pyridinemethanol
3-Pyridinemethanol is a useful synthetic intermediate. It can be used in the synthesis of histone deacetylase inhibitors. It can also be used to prepare (phenyl)(cyanopyrazinyl)urea derivatives as possible kinase-1 (Chk1) inhibitors.
What are the applications of Application
3-Pyridinemethanol is used in the synthesis of histone deacetylase inhibitors
Definition
ChEBI: 3-pyridinemethanol is a member of the class of pyridines that is pyridine which is substituted by a hydroxymethyl group at position 3. It has a role as a vasodilator agent and an antilipemic drug. It is a member of pyridines and an aromatic primary alcohol.
Manufacturing Process
The catalyst is prepared by suspending 5 kg of catalyst grade charcoal in 200
liters of water, in a pressure vessel, and adding thereto 25 liters of 4% (as Pd
metal) aqueous palladous chloride. Air is displaced from the vessel and then
hydrogen is passed into the aqueous mixture at a pressure of 3 to 5 psi, while
stirring, until no further absorption is noted and the chloride is completely
reduced to metal.
To the aqueous suspension of the palladized charcoal catalyst thus obtained
are added 20.8 kg of 3-cyano-pyridine (96% purity); and then are added 70 liters of a hydrochloric acid solution prepared by diluting 30 liters of 36% HCl
with 40 liters of water. This represents approximately 1.75 mols of HCl for
each mol of 3-cyano-pyridine. The suspension is maintained at 10° to 15°C
and stirred continuously while introducing a current of hydrogen at a pressure
of 3 to 5 psi. When absorption of hydrogen ceases and the 3-cyanopyridine is
completely reduced, the reaction mixture is filtered to remove the catalyst.
The filter cake is washed with 40 liters of water in two equal portions, and the
wash water is added to the filtrate.
The combined liquors, which comprise an aqueous hydrochloric acid solution
of 3-aminomethyl-pyridine hydrochloride, are then heated to a temperature of
60° to 65°C, and ethyl nitrite gas is passed into the heated solution. The ethyl
nitrite is generated by placing 20 liters of 90% ethyl alcohol in a suitable
vessel, diluting with 200 liters of water, and, while stirring, adding to the
dilute alcohol 18.3 kg of nitrosyl chloride at the rate of 2.25 kg per hour. (The
process using methyl nitrite is carried out by substituting a stoichiometrically
equivalent quantity of methyl alcohol for the ethyl alcohol.)
When all the ethyl nitrite has been added, the reaction mixture is refluxed for
approximately one hour, then concentrated to dryness under reduced pressure
(25 to 30 mm Hg) and at a maximum temperature of 70°C. The crystalline
residue is dissolved in 35 liters of water and adjusted to a pH of 8 to 9 by
addition (with cooling and stirring) of 11 to 12 kg of caustic soda. The sodium
chloride formed is filtered off, and the filter cake is washed with 20 liters of
normal butyl alcohol. This wash liquid is used for the first extraction of the
product from the aqueous filtrate. The filtrate is then further extracted with
four successive 20-liter portions of n-butyl alcohol.
All the extracts are combined and concentrated in vacuo (100°C/20 mm) to
remove the n-butyl alcohol. The residue is submitted to fractionation under
reduced pressure. The forerun (up to 112°C/2 to 3 mm) consists of a small
amount of n-butyl alcohol and some 3-pyridylcarbinol. The main fraction,
boiling at 112° to 114°C/2 to 3 mm, consists of 3-pyridylcarbinol.
brand name
Roniacol (HoffmannLaRoche).
Therapeutic Function
Vasodilator
Synthesis Reference(s)
The Journal of Organic Chemistry, 28, p. 3261, 1963 DOI: 10.1021/jo01046a538
Synthesis, p. 55, 1987
General Description
3-Pyridinemethanol, an aromatic primary alcohol, is the key moiety of many bio-active and industrially important compounds.
Flammability and Explosibility
Not classified
Properties of 3-Pyridinemethanol
| Melting point: | -7 °C |
| Boiling point: | 154 °C/28 mmHg (lit.) |
| Density | 1.124 g/mL at 25 °C (lit.) |
| vapor pressure | 0.342Pa at 20℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | 1000mg/l |
| form | liquid (clear) |
| pka | 13.68±0.10(Predicted) |
| color | clear light yellow |
| PH | 7-8 (100g/l, H2O, 20℃) |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Merck | 14,6527 |
| BRN | 107851 |
| CAS DataBase Reference | 100-55-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Nicotinyl Alcohol(100-55-0) |
| EPA Substance Registry System | 3-Pyridinemethanol (100-55-0) |
Safety information for 3-Pyridinemethanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 3-Pyridinemethanol
| InChIKey | MVQVNTPHUGQQHK-UHFFFAOYSA-N |
3-Pyridinemethanol manufacturer
SGT life sciences
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

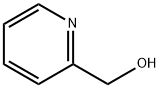

You may like
-
 100-55-0 3-Pyridine methanol 99%View Details
100-55-0 3-Pyridine methanol 99%View Details
100-55-0 -
 Nicotinyl alcohol 98%View Details
Nicotinyl alcohol 98%View Details
100-55-0 -
 100-55-0 Nicotinyl alcohol 98%View Details
100-55-0 Nicotinyl alcohol 98%View Details
100-55-0 -
 3-Pyridinemethanol CAS 100-55-0View Details
3-Pyridinemethanol CAS 100-55-0View Details
100-55-0 -
 3-(Hydroxymethyl)pyridine CAS 100-55-0View Details
3-(Hydroxymethyl)pyridine CAS 100-55-0View Details
100-55-0 -
 3-Pyridinemethanol, 98% CAS 100-55-0View Details
3-Pyridinemethanol, 98% CAS 100-55-0View Details
100-55-0 -
 3-Pyridinemethanol CAS 100-55-0View Details
3-Pyridinemethanol CAS 100-55-0View Details
100-55-0 -
 100-55-0 98+View Details
100-55-0 98+View Details
100-55-0
