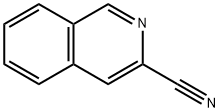
3-Methylisoquinoline
- CAS NO.:1125-80-0
- Empirical Formula: C10H9N
- Molecular Weight: 143.19
- MDL number: MFCD00024139
- EINECS: 214-412-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
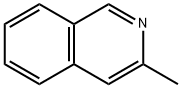
What is 3-Methylisoquinoline?
Description
The metabolites of 3-methylisoquinoline were separated by adsorption and reversed-phase high-performance liquid chromatography (HPLC).
The Uses of 3-Methylisoquinoline
3-Methylisoquinoline was used to prepare 3-aminoisoquinoline.
Preparation
Benzylamine (9.18 mL, 84.0 mmol) and 1,1-dimethoxypropan-2-one (9.95 mL, 84.0 mmol) were added to dichloromethane (350 mL) at room temperature. Sodium triacetoxyborohydride (25 g, 118 mmol) was added to the reaction mixture in one portion. The reaction was stirred at room temperature overnight. The reaction mixture was then diluted with 2.5% sodium bicarbonate (250 mL) and mixed for 30 minutes, becoming biphasic. The organic layer was discarded. The aqueous layer was basified to pH 14 using concentrated sodium hydroxide. The basified aqueous solution was washed three times with ethyl acetate and the organic layers were kept and combined. Then, the resulting solution was washed with 5% sodium chloride solution three times. The organic layers were combined and dried over sodium sulfate. The resulting solution was evaporated to a yellow oil, which was found to be N-benzyl-1,1-dimethoxypropan-2-one. The oil (2.62 g, 12.5 mmol) was added dropwise to chlorosulfonic acid (8.35 mL, 125 mmol) over ice. A few milliliters of dichloromethane was used to rinse the oil from its reaction flask and the oil dissolved in dichloromethane was also added to the chlorosulfonic acid flask over ice. The reaction mixture was placed over boiling water for 5 minutes with a condenser to evaporate the solvent used to rinse the N-benzyl-1,1-dimethoxypropan-2-one out of its previous flask while not letting any water vapor enter the flask before the reaction mixture was fully heated. Then, the reaction mixture was placed in the boiling water for 10 minutes without a condenser to allow methanol, a side-product of the reaction, to evaporate out of the flask and drive the reaction forward. After cooling, the reaction mixture was quenched with ice and then basified to pH 14 with concentrated sodium hydroxide. The mixture was washed with dichloromethane three times. The organic layers were combined and dried using magnesium sulfate. The resulting solution was evaporated to solid 3-methylisoquinoline (average percent yield: 2-5%) and the structure was confirmed with GC-MS and NMR.
Definition
ChEBI: 3-methylisoquinoline is an isoquinoline substituted by a methyl group at position 3. It has a role as a bacterial xenobiotic metabolite.
Synthesis Reference(s)
Tetrahedron Letters, 26, p. 3959, 1985 DOI: 10.1016/S0040-4039(00)98697-0
Chemical and Pharmaceutical Bulletin, 35, p. 4964, 1987 DOI: 10.1248/cpb.35.4964
Properties of 3-Methylisoquinoline
| Melting point: | 63-65 °C(lit.) |
| Boiling point: | 251 °C(lit.) |
| Density | 1.0584 (estimate) |
| refractive index | 1.6152 (estimate) |
| pka | 5.66±0.30(Predicted) |
| Water Solubility | 919mg/L(20 ºC) |
| CAS DataBase Reference | 1125-80-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Isoquinoline, 3-methyl-(1125-80-0) |
| EPA Substance Registry System | Isoquinoline, 3-methyl- (1125-80-0) |
Safety information for 3-Methylisoquinoline
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Methylisoquinoline
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 3-Methylisoquinoline CAS 1125-80-0View Details
3-Methylisoquinoline CAS 1125-80-0View Details
1125-80-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8