3-Aminobenzotrifluoride
Synonym(s):α,α,α-Trifluoro-m-toluidine;3-Aminobenzotrifluoride
- CAS NO.:98-16-8
- Empirical Formula: C7H6F3N
- Molecular Weight: 161.12
- MDL number: MFCD00014717
- EINECS: 202-643-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
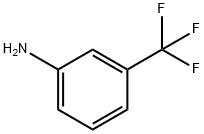
What is 3-Aminobenzotrifluoride?
Description
Benzenamine, 3-(trifluoromethyl)- is a combustible, colorless to yellow oily liquid with an amine-likeodor. Molecular weight=161.14; Boiling point = 187.5℃;Freezing/Melting point= 3℃; Flash point =85℃.Hazard Identification (based on NFPA-704 M RatingSystem): Health 2, Flammability 1, Reactivity 0.
Chemical properties
Clear light yellow liquid
Chemical properties
Benzenamine, 3-(trifluoromethyl)-is a combustible, colorless to yellow oily liquid. Unpleasant amine odor.
The Uses of 3-Aminobenzotrifluoride
Pharmaceutical intermediate.
The Uses of 3-Aminobenzotrifluoride
Used as pharmaceutical intermediate.
General Description
A colorless liquid with a fishlike odor. Insoluble in water and denser than water. Toxic by ingestion and inhalation. Used to make dyes and pharmaceuticals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
3-Aminobenzotrifluoride is a halogenated amine derivative. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Health Hazard
Contact may cause burns to skin and eyes. May be poisonous if inhaled, swallowed or absorbed through the skin.
Fire Hazard
3-Aminobenzotrifluoride may burn but does not ignite readily. Cylinder may explode in heat of fire. When heated to decomposition, 3-Aminobenzotrifluoride emits very toxic fumes of fluorides and nitrogen oxides.
Safety Profile
Poison by inhalation, ingestion, and intraperitoneal routes. May be moderately toxic by other routes. See also AMINES and FLUORIDES. When heated to decomposition it emits very toxic fumes of Fand NOx,.
Potential Exposure
This material is used as a chemical intermediate for herbicides, antihypertensives, and diuretics.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Keepvictim quiet and maintain normal body temperature. Effectsmay be delayed; keep victim under observation.
Storage
Color Code—Green: General storage may be used.Store in tightly closed, light-resistant containers in a cool,well-ventilated area away from oxidizers. Sources of ignition, such as smoking and open flames, are prohibitedwhere this chemical is used, handled, or stored in a mannerthat could create a potential fire or explosion hazard.
Shipping
UN29483-Trifluoromethylaniline, Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Light and air sensitive.
Properties of 3-Aminobenzotrifluoride
| Melting point: | 5 °C |
| Boiling point: | 187 °C(lit.) |
| Density | 1.29 g/mL at 25 °C(lit.) |
| vapor pressure | 0.3 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 185 °F |
| storage temp. | Store below +30°C. |
| solubility | 5 g/L (20°C) |
| form | Liquid |
| pka | 3.49(at 25℃) |
| Specific Gravity | 1.290 |
| color | Clear light yellow |
| Water Solubility | 5 g/L (20 ºC) |
| BRN | 387672 |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| CAS DataBase Reference | 98-16-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenamine, 3-(trifluoromethyl)-(98-16-8) |
| EPA Substance Registry System | m-(Trifluoromethyl)aniline (98-16-8) |
Safety information for 3-Aminobenzotrifluoride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Aminobenzotrifluoride
| InChIKey | VIUDTWATMPPKEL-UHFFFAOYSA-N |
3-Aminobenzotrifluoride manufacturer
JSK Chemicals
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 98-16-8 99%View Details
98-16-8 99%View Details
98-16-8 -
 3-Aminobenzotrifluoride, 99% 99%View Details
3-Aminobenzotrifluoride, 99% 99%View Details
98-16-8 -
 3-Aminobenzotrifluoride CAS 98-16-8View Details
3-Aminobenzotrifluoride CAS 98-16-8View Details
98-16-8 -
 3-Aminobenzotrifluoride, 98% CAS 98-16-8View Details
3-Aminobenzotrifluoride, 98% CAS 98-16-8View Details
98-16-8 -
 3-(Trifluoromethyl)aniline CAS 98-16-8View Details
3-(Trifluoromethyl)aniline CAS 98-16-8View Details
98-16-8 -
 3 -Amino benzotrifluoride (98-16-8)View Details
3 -Amino benzotrifluoride (98-16-8)View Details
98-16-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
