3-(acetylamino)propanesulphonic acid
- CAS NO.:77337-76-9
- Empirical Formula: C5H11NO4S
- Molecular Weight: 181.21
- MDL number: MFCD00867423
- EINECS: 278-667-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
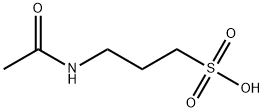
What is 3-(acetylamino)propanesulphonic acid?
Absorption
Acamprosate is absorbed in the gastrointestinal tract. The absolute bioavailability of acamprosate after oral administration is about 11%. The effect of food absorption is clinically insignificant and no adjustment of the dose is necessary with regard to meals. After repeated oral doses of 666 mg 3 times a day, steady-state concentrations are achieved within 5 to 7 days, with plasma concentration ranging between 370 to 650 micrograms/L.
Toxicity
The intraperitoneal LD50 in male mice is 1.87 g/kg. In reported cases of acute overdosage with acamprosate (doses of up to 56 grams of acamprosate calcium) diarrhea was the only reported symptom attributable to acamprosate. In the case of an overdose, supportive and symptomatic treatment is recommended.
Originator
Campral,Merck
The Uses of 3-(acetylamino)propanesulphonic acid
Treatment of alcohol dependency.
Background
Alcohol use disorder is responsible for a large worldwide burden of morbidity, premature mortality, and economic consequences resulting from accidents, violence, incarceration, decreased productivity, and increased healthcare spending.
Acamprosate, also known by the brand name Campral, is a drug used for the maintenance of alcohol abstinence. It is a structural analogue of the neurotransmitter γ-aminobutyric acid (GABA). Acamprosate is the first medication specifically formulated for the maintenance of alcohol abstinence in ethanol-dependent patients after alcohol detoxification, unlike naltrexone and disulfiram. It was first approved by the FDA in 2004 and initially marketed by Forest Laboratories.
Indications
Acamprosate is indicated for the maintenance of abstinence from alcohol in patients with alcohol dependence who are abstinent at treatment initiation. It is also indicated for the maintenance of alcohol abstinence in patients who have undergone alcohol detoxification. This drug should be used with a psychosocial support program providing adequate support.
Definition
ChEBI: An organosulfonic acid that is propane-1-sulfonic acid substituted by an acetylamino group at position 3.
Manufacturing Process
In a 4 liter flask provided with stirring means, a bromine funnel and a
thermometer, 17.5% sodium hydroxide solution and aminopropanesulfonic
acid (homotaurine) are added.
After complete dissolution, at a temperature of between 25°-40°C, acetic
anhydride are added so as not to exceed a temperature of between 30°-40°C.
The mixture is then maintained at this temperature by heating for at least 1
h.
The solution is then concentrated in vacuum, the residue is redissolved in 2.5
L of distilled water and the mixture is concentrated again. The residue is then
dissolved in 1.6 L of distilled water, filtered, then concentrated almost
completely. Drying is terminated in an oven in vacuum. A colorless crystalline
powder of 3-acetylaminopropanesulfonate of sodium (sodium Nacetylhomotaurinate)
is obtained.
The 3-acetylaminopropanesulfonic acid may be produced by treatment of 3-
acetylaminopropanesulfonate of sodium with hydrochloric acid.
In practice it is usually used as calcium salt.
brand name
Campral (Forest).
Therapeutic Function
Psychotropic
Pharmacokinetics
Acamprosate acts on the CNS, aiding in the restoration of normal glutaminergic neuron activity. Pharmacodynamic studies have shown that acamprosate calcium reduces alcohol intake in alcohol-dependent individuals, likely through effects on NMDA receptors and calcium channels. It is a safe and well-tolerated drug for patients with alcohol dependency and improves the likelihood of alcohol abstinence.
Metabolism
Acamprosate is not metabolized.
Safety information for 3-(acetylamino)propanesulphonic acid
3-(acetylamino)propanesulphonic acid manufacturer
Alfa Omega Pharma
Aarti Drugs Ltd (part of Aarti Group of Industries)
KPS Chemicals And Pharmaceuticals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



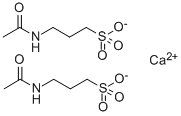
You may like
-
 77337-76-9 Acamprosate 98%View Details
77337-76-9 Acamprosate 98%View Details
77337-76-9 -
 77337-76-9 98%View Details
77337-76-9 98%View Details
77337-76-9 -
 77337-76-9 98%View Details
77337-76-9 98%View Details
77337-76-9 -
 Acamprosate 99%View Details
Acamprosate 99%View Details -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1