Potassium sorbate
- CAS NO.:24634-61-5
- Empirical Formula: C6H7KO2
- Molecular Weight: 150.22
- MDL number: MFCD00016546
- EINECS: 246-376-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:16
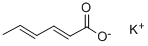
What is Potassium sorbate?
Description
Potassium sorbate is a white crystalline powder. It is a potassium salt of sorbic acid.It was originally discovered in the 1850’s, and was derived from the Mountain Ash Tree. Today, potassium sorbate is synthetically created.Potassium sorbate is a good food preservatives, fully degradable, similar to fatty acids found naturally in foods. It is used to slow the growth of molds and yeasts in foods. It is commonly found in margarine, wines, cheeses, yogurts, soft drinks, and baked goods. Potassium sorbate has been used has a food preservative for many years. There have been extensive long-term tests that have confirmed its safety and it is on the Center for Science in the Public Interest list of safe additives.
Chemical properties
Potassium sorbate occurs as a white crystalline powder with a faint, characteristic odor.
Chemical properties
Potassium sorbate is the potassium salt of sorbic acid, chemical formula C6H7KO2. Its primary use is as a food preservative (E number 202). Potassium sorbate is effective in a variety of applications including food, wine, and personal care products. Commercial sources are now produced by the condensation of crotonaldehyde and ketene (Ashford, 1994).
Chemical properties
Potassium sorbate is produced by neutralizing potassium hydroxide with sorbic acid, an unsaturated carboxylic acid that occurs naturally in some berries. The colourless salt is very soluble in water (58.2% at 20°C).
Physical properties
Colorless or white crystalline solid; density 1.36 g/cm3; decomposes at 270°C; soluble in water, 58 g/100 g solution; moderately soluble in alcohol.
The Uses of Potassium sorbate
Sorbic acid and its potassium salt is commonly employed as food preservative in wide range of foodstuffs, such as cheese, pickles, sauces and wines.
Potassium sorbate is a food grade preservative generally regarded as safe (GRAS) worldwide. It is the inactive salt of sorbic acid. It readily dissolves in water where it converts to sorbic acid, its active form, at a low pH. Sorbic acid is very pH dependent. While it shows some activity up to pH 6 (about 6%), it is most active at pH 4.4 (70%). At pH 5.0 it is 37% active. As sorbic acid, it is considered to be active against mold, fair against yeast and poor against most bacteria. Sorbic acid is an unsaturated fatty acid and as such is subject to oxidation (use of an antioxidant like Mixed Tocopherols T50 is recommended). It is also sensitive to UV light and may turn yellow in solution. Gluconolactone is reported to stabilize potassium sorbate against discoloration and darkening in aqueous solutions and may be useful in stabilizing sorbic acid in the water phase of a product.
The Uses of Potassium sorbate
Sorbic acid is a naturally occurring polyunsaturated fat that has antimicrobial properties. That means that it helps to prevent the growth of molds, yeasts, and fungus. Potassium sorbate is found in many food products, especially those which are meant to be stored and eaten at room temperature. This helps to ward off particles such as mold or fungus that can cause foods to spoil or make people sick. Baked goods, processed fruits and vegetables or dairy products frequently contain this product. When brewing wine, yeast is used to convert sugar to alcohol. This process is called fermentation. When the wine reaches the desired flavour and body, you want to stop the yeast from growing. Potassium sorbate is added to inhibit yeast growth.
The Uses of Potassium sorbate
As mold and yeast inhibitor, like sorbic acid, especially where greater soly in water is desirable.
The Uses of Potassium sorbate
Potassium sorbate is used to inhibit molds and yeasts in many foods, such as cheese, wine, yogurt, dried meats, apple cider, soft drinks and fruit drinks, and baked goods. It can also be found in the ingredients list of many dried fruit products. In addition, herbal dietary supplement products generally contain potassium sorbate, which acts to prevent mold and microbes and to increase shelf life, and is used in quantities at which there are no known adverse health effects, over short periods of time.Labeling of this preservative on ingredient statements reads as "potassium sorbate" and or "E202". Also, it is used in many personal care products to inhibit the development of microorganisms for shelf stability. Some manufacturers are using this preservative as a replacement for parabens.
Also known as "wine stabilizer", potassium sorbate produces sorbic acid when added to wine. It serves two purposes. When active fermentation has ceased and the wine is racked for the final time after clearing, potassium sorbate will render any surviving yeast incapable of multiplying. Yeast living at that moment can continue fermenting any residual sugar into CO2 and alcohol, but when they die no new yeast will be present to cause future fermentation.
What are the applications of Application
Potassium sorbate is a mold and yeast inhibitor.
Definition
ChEBI: A potassium salt having sorbate as the counterion.
Production Methods
Potassium sorbate is prepared from sorbic acid and potassium hydroxide.
Production Methods
Potassium sorbate is produced by reacting sorbic acid with an equimolar portion of potassium hydroxide. The resulting potassium sorbate may be crystallized from aqueous ethanol.
Most of the sorbic acid is generally prepared by a process comprising the steps of reacting crotonaldehyde with ketene in the presence of a catalyst (e.g., a fatty acid salt of zinc) to yield a polyester, and hydrolyzing the polyester with an acid or an alkali, or decomposing the polyester in a hot water.
Preparation
Potassium sorbate is prepared by reacting potassium hydroxide with sorbic acid, followed by evaporation and crystallization:CH3CH=CHCH=CHCOOH + KOH →CH3CH=CHCH=CHCOOK + H2O.
General Description
Sorbates have been reported to be less toxic than benzoate and have been classified as “Generally Recognized as Safe” (GRAS) additives by the U.S. Food and Drug Administration (FDA).Sorbic acid is metabolised to mainly to carbon dioxide. While the minor amounts are converted to trans,trans-muconic acid (ttMA), which is excreted unchanged into the urine. Urinary ttMA is a biomarker for the occupational and environmental exposure to benzene. Ability of trans,trans-2,4-Hexadienoic acid potassium salt (Sorbic acid potassium salt, Potassium sorbate) to induce chromosome aberrations, sister chromatid exchanges (SCE) and gene mutations in cultured Chinese hamster V79 cells has been examined.Potassium sorbate is reported to be less genotoxic than the sodium salt analog.
Pharmaceutical Applications
Potassium sorbate is an antimicrobial preservative, with antibacterial
and antifungal properties used in pharmaceuticals, foods, enteral
preparations, and cosmetics. Generally, it is used at concentrations
of 0.1–0.2% in oral and topical formulations, especially those
containing nonionic surfactants. Potassium sorbate has been used to
enhance the ocular bioavailability of timolol.
Potassium sorbate is used in approximately twice as many
pharmaceutical formulations as is sorbic acid owing to its greater solubility and stability in water. Like sorbic acid, potassium sorbate
has minimal antibacterial properties in formulations above pH 6.
Toxicology
Potassium sorbate is a skin, eye and respiratory irritant. Although some research implies it has a long term safety record, in vitro studies have shown that it is both genotoxic and mutagenic to human blood cells. Potassium sorbate is found to be toxic to human DNA in peripheral blood lymphocytes (type of white blood cells), and hence found that it negatively affects immunity. It is often used with ascorbic acid and iron salts as they increase its effectiveness but this tends to form mutagenic compounds that damage DNA molecules.
Potassium sorbate exhibits low toxicity with LD50 (rat, oral) of 4.92 g / kg, similar to that of table salt . Typical usage rates of potassium sorbate are 0.025 % to 0.1 % (see sorbic acid), which in a 100 g serving yields intake of 25 mg to 100 mg. Acceptable daily intakes for human is 12.5 mg / kg, or 875 mg daily for an average adult (70 kg), according to FAO/World Health Organization Expert Committee on Food Additives.
Safety
Potassium sorbate is used as an antimicrobial preservative in oral
and topical pharmaceutical formulations and is generally regarded
as a relatively nontoxic material. However, some adverse reactions
to potassium sorbate have been reported, including irritant skin
reactions which may be of the allergic, hypersensitive type. There
have been no reports of adverse systemic reactions following oral
consumption of potassium sorbate.
The WHO has set an estimated total acceptable daily intake for
sorbic acid, calcium sorbate, potassium sorbate, and sodium
sorbate expressed as sorbic acid at up to 25 mg/kg body-weight.
(mouse, IP): 1.3 g/kg
(rat, oral): 4.92 g/kg
See also Sorbic Acid.
Storage
Potassium sorbate is more stable in aqueous solution than sorbic
acid; aqueous solutions may be sterilized by autoclaving.
The bulk material should be stored in a well-closed container,
protected from light, at a temperature not exceeding 40°C.
Incompatibilities
Some loss of antimicrobial activity occurs in the presence of nonionic surfactants and some plastics.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (nasal sprays; oral capsules, solutions, suspensions, syrups, tablets; topical creams and lotions). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Potassium sorbate
| Melting point: | 270 °C |
| Density | 1,361 g/cm3 |
| vapor pressure | <1 Pa (20 °C) |
| FEMA | 2921 | POTASSIUM SORBATE |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless to faintly yellow |
| form | Powder |
| pka | 4.69[at 20 ℃] |
| color | White to light cream |
| Odor | Odorless |
| PH Range | 8 - 11 at 580 g/l at 20 °C |
| PH | 7.8 (H2O, 20.1℃) |
| Water Solubility | 58.2 g/100 mL (20 ºC) |
| Merck | 14,7671 |
| BRN | 5357554 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 24634-61-5(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium sorbate (24634-61-5) |
Safety information for Potassium sorbate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium sorbate
| InChIKey | CHHHXKFHOYLYRE-STWYSWDKSA-M |
Potassium sorbate manufacturer
JSK Chemicals
Innovative
Atharva Trading & Company
Pachisia Chemical Works
JKM Chemtrade
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



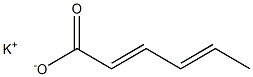
You may like
-
 Potassium Sorbate (SQ) CAS 24634-61-5View Details
Potassium Sorbate (SQ) CAS 24634-61-5View Details
24634-61-5 -
 Potassium Sorbate CASView Details
Potassium Sorbate CASView Details -
 Potassium sorbate 99% (HPLC) CAS 24634-61-5View Details
Potassium sorbate 99% (HPLC) CAS 24634-61-5View Details
24634-61-5 -
 Potassium sorbate 95% CAS 24634-61-5View Details
Potassium sorbate 95% CAS 24634-61-5View Details
24634-61-5 -
 Potassium Sorbate pure CAS 24634-61-5View Details
Potassium Sorbate pure CAS 24634-61-5View Details
24634-61-5 -
 Potassium Sorbate Food Grade, CAS Number: 24634-61-5, Chemical Formula: C6H7KO2View Details
Potassium Sorbate Food Grade, CAS Number: 24634-61-5, Chemical Formula: C6H7KO2View Details
24634-61-5 -
 White To Off White Potassium Sorbate (CAS Number: 24634-61-5), Packaging Size: 25 KgView Details
White To Off White Potassium Sorbate (CAS Number: 24634-61-5), Packaging Size: 25 KgView Details
24634-61-5 -
 Potassium Sorbate Chemical, For Industrial, Packaging Type: BagView Details
Potassium Sorbate Chemical, For Industrial, Packaging Type: BagView Details
24634-61-5
