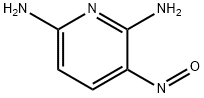
2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-
Synonym(s):GSK-3 Inhibitor XXIX, CHIR98014 - CAS 252935-94-7 - Calbiochem;
- CAS NO.:252935-94-7
- Empirical Formula: C20H17Cl2N9O2
- Molecular Weight: 486.31
- MDL number: MFCD10565922
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
![2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO- Structural](https://img.chemicalbook.in/CAS/GIF/252935-94-7.gif)
What is 2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-?
The Uses of 2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-
CHIR 98014 has been used for the generation of small molecules neural progenitor cells and differentiation towards motor neurons. It has also been used as a Wnt/β-catenin pharmacological agonist in the cell-conditioned medium to perform chromatin immunoprecipitation (ChIP) studies in HT22 neurons.
What are the applications of Application
CHIR-98014 is a selective and ATP-competitive inhibitor of GSK-3β
Definition
ChEBI: CHIR-98014 is a member of the class of aminopyrimidines that is pyrimidine substituted by {2-[(6-amino-5-nitropyridin-2-yl)amino]ethyl}amino, 2,4-dichlorophenyl, and 1H-imidazol-1-yl groups at positions 2, 4 and 5, respectively. It is a potent ATP-competitive inhibitor of GSK3alpha and GSK3beta (IC50 values of 0.65 and 0.58 nM, respectively). It has a role as an EC 2.7.11.26 (tau-protein kinase) inhibitor, an apoptosis inducer, an antineoplastic agent, a hypoglycemic agent, a Wnt signalling activator and a tau aggregation inhibitor. It is a secondary amino compound, a dichlorobenzene, a member of imidazoles, a diaminopyridine, an aminopyrimidine and a C-nitro compound.
Biological Activity
chir-98014 is a potent inhibitor of gsk-3α and gsk-3β with ic50 values of 0.65 nm and 0.58 nm, respectively [1]gsk-3 (glycogen synthase kinase 3) is a serine/threonine protein kinase and plays a pivotal role in a number of central intracellular signaling pathways, including cellular proliferation, migration, inflammation and immune responses, glucose regulation, and apoptosis. recently, it has been reported that gsk-3 abnormally expressed in a variety of diseases, including type ii diabetes, alzheimer's disease, inflammation, cancer, and bipolar disorder [2, 3].chir-98014 is a potent gsk-3α and gsk-3β inhibitor. when tested with insulin receptor-expressing cho-ir cells or primary rat hepatocytes, chir-98014 stimulated the gs activity ratio as high as two- to three fold compared with basal in a dose dependent manner. similarly, in isolated type 1 skeletal muscle from insulin-sensitive lean zucker and from insulin-resistant zdf rats, administration of chir-98014 activated gs activity ratio [1]. in mouse es-d3 cells, chir-98014 treatment (48 and 72 hours later) resulted in a significant activation of the wnt/beta-catenin pathway via inhibiting gsk-3 [4].in markedly diabetic and insulin-resistant db/db mice model, oral administration of chir-98014 (30mg/kg) significantly reduced fasting hyperglycemia within 4 hours and improved glucose disposal during an ipgtt [1].
Biochem/physiol Actions
CHIR 98014 is a glycogen synthase kinase-3 (GSK-3) inhibitor with IC50 values of 0.65 nM and 0.58 nM for GSK3α and GSK3β, respectively. CHIR98014 is 500-fold to >10,000-fold selectivity for GSK-3 versus 20 other protein kinases tested, including closest homologs Cdc2 and ERK2.
storage
Store at -20°C
References
[1]. ring, d.b., et al., selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. diabetes, 2003. 52(3): p. 588-95.
[2]. pan wa, et al. the rna recognition motif of nifk is required for rrna maturation during cell cycle progression. rna biol. 2015. 12(3):255-67.
[3]. mccubrey ja, et al. gsk-3 as potential target for therapeutic intervention in cancer. oncotarget. 2014. 5(10):2881-911.
[4]. naujok o, et al. cytotoxicity and activation of the wnt/beta-catenin pathway in mouse embryonic stem cells treated with four gsk3 inhibitors. bmc res notes. 2014. 7(1):273-281.
Properties of 2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-
| Density | 1.62 |
| storage temp. | -20°C |
| solubility | insoluble in H2O; insoluble in EtOH; ≥8.1 mg/mL in DMSO with gentle warming |
| form | powder |
| color | white to brown |
Safety information for 2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-
Computed Descriptors for 2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-](https://img.chemicalbook.in/CAS/GIF/252935-94-7.gif)
You may like
-
 GSK-3 Inhibitor XXIX, CHIR98014 CAS 252935-94-7View Details
GSK-3 Inhibitor XXIX, CHIR98014 CAS 252935-94-7View Details
252935-94-7 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1