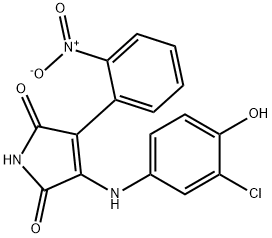
SB 415286
Synonym(s):3-[(3-Chloro-4-hydroxyphenyl)-amino]-4-(2-nitrophenyl)-1H-pyrrol-2,5-dione
- CAS NO.:264218-23-7
- Empirical Formula: C16H10ClN3O5
- Molecular Weight: 359.72
- MDL number: MFCD04039789
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
What is SB 415286?
Description
Glycogen synthase kinase 3 (GSK3) is a serine/threonine protein kinase that is inhibited by an assortment of extracellular stimuli such as insulin, growth factors, cell specification factors, and cell adhesion. Its activity regulates many cell functions including the control of cell division, apoptosis, and inflammation. SB-415286 is a potent and selective cell-
The Uses of SB 415286
SB 415286 was used to treat neuroblastoma cells and study the effect of GSK-3 inhibition on cell proliferation.
What are the applications of Application
SB-415286 is an ATP-competitive inhibitor of GSK3α and GSK-3β
Definition
ChEBI: A member of the class of maleimides carrying 3-chloro-4-hydroxyphenylamino and 2-nitrophenyl substituents at positions 3 and 4 respectively.
Biological Activity
Potent and selective glycogen synthase kinase-3 (GSK-3) inhibitor (K i = 31 nM for GSK-3 α ); competes with ATP. Has minimal activity against 24 other protein kinases (IC 50 > 10 μ M). Stimulates glycogen synthesis, gene transcription and is neuroprotective.
Biochem/physiol Actions
SB 415286 is a small molecule inhibitor of GSK-3 in muscle and fat cells. SB 415286 induces activation of glycogen synthase and regulates the transport glucose. SB 415286 reduces the systemic inflammation induced by endotoxic shock in rat model of acute colitis. It increases the axonal growth and promotes the recovery of injured adult CNS neurons. SB 415289 is implicated in inducing chromosome instability when used as therapeutic agents.
Storage
Store at RT
References
selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. chem biol. 2000 oct;7(10):793-803.selective small-molecule inhibitors of glycogen synthase kinase-3 activity protect primary neurones from death. j neurochem. 2001 apr;77(1):94-102.role of glycogen synthase kinase 3beta in rapamycin-mediated cell cycle regulation and chemosensitivity. cancer res. 2005 mar 1;65(5):1961-72.pharmacologic modulation of glycogen synthase kinase-3beta promotes p53-dependent apoptosis through a direct bax-mediated mitochondrial pathway in colorectal cancer cells. cancer res. 2005 oct 1;65(19):9012-20.glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. circ res. 2008 oct 24;103(9):983-91. doi: 10.1161/circresaha.108.178970. epub 2008 sep 18.glycogen synthase kinase 3β inhibitors protect hippocampal neurons from radiation-induced apoptosis by regulating mdm2-p53 pathway. cell death differ. 2012 mar;19(3):387-96. doi: 10.1038/cdd.2011.94. epub 2011 jul 8.a gsk-3β inhibitor protects against radiation necrosis in mouse brain. int j radiat oncol biol phys. 2014 jul 15;89(4):714-21. doi: 10.1016/j.ijrobp.2014.04.018.
Properties of SB 415286
| Boiling point: | 595.8±50.0 °C(Predicted) |
| Density | 1.647 |
| storage temp. | -20°C |
| solubility | DMSO: 16 mg/mL |
| form | Yellow Solid. |
| pka | 6.81±0.60(Predicted) |
| color | yellow to orange |
Safety information for SB 415286
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SB 415286
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![2,6-PYRIDINEDIAMINE, N6-[2-[[4-(2,4-DICHLOROPHENYL)-5-(1H-IMIDAZOL-1-YL)-2-PYRIMIDINYL]AMINO]ETHYL]-3-NITRO-](https://img.chemicalbook.in/CAS/GIF/252935-94-7.gif)


![2-THIO(3-IODOBENZYL)-5-(1-PYRIDYL)-[1,3,4]-OXADIAZOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB2201711.gif)
You may like
-
 SB 415286 98.00% CAS 264218-23-7View Details
SB 415286 98.00% CAS 264218-23-7View Details
264218-23-7 -
 SB 415286 CAS 264218-23-7View Details
SB 415286 CAS 264218-23-7View Details
264218-23-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
