2,4-Dichlorophenoxyacetic acid
Synonym(s):2,4 D-acid;2,4-D;2,4-Dichlorophenoxyacetic acid;2,4-Dichlorophenoxyacetic acid solution
- CAS NO.:94-75-7
- Empirical Formula: C8H6Cl2O3
- Molecular Weight: 221.04
- MDL number: MFCD00004300
- EINECS: 202-361-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
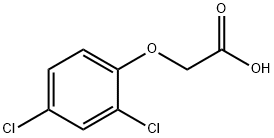
What is 2,4-Dichlorophenoxyacetic acid?
Description
2,4-D free acids, esters, amines, and salts are formulated in water suspensions or solutions, or in various organic solvents, for application as systemic herbicides that are used postemergence for selective control of broadleaf weeds.
Description
2,4-Dichlorophenoxyacetic acid—“2,4-D” to millions of farmers—is one of the most widely used broadleaf herbicides in the world. Its preparation from 2,4-dichlorophenol and chloroacetic acid was?reported by R. Pokorny in 1941, and it was developed as a major herbicide during World War II. Recently, EPA, in response to a petition from the Natural Resources Defense Council to ban 2-4D, declared that?its approved uses are safe.
Chemical properties
2,4-Dichlorophenoxyacetic acid is a white to yellow crystalline powder with a slight phenolic odor. It is used as a herbicide for the selective control of broad-leaved weeds in agriculture, and for the control of woody plants along roadsides, railways, and utilities rights of way. It is one of the most widely used herbicides in the world and is commonly used on crops such as wheat and corn, and on pasture and rangelands. It is also used to control broad-leaved aquatic weeds.
Physical properties
Odorless, white to pale yellow, powder or prismatic crystals. Impure formulations containing 2,4- D as the main component may have a phenolic odor.
The Uses of 2,4-Dichlorophenoxyacetic acid
2,4-Dichlorophenoxyacetic acid is often formulated as various forms of inorganic salts or esters. 2,4-D was first registered as a herbicide in 1948, and its annual production was estimated at 52–67 million lb in 1990. The primary use of 2,4-D is for control of broadleaf weeds, and as such, it is used for a large spectrum of applications in agriculture, forestry, and lawn care. 2,4-D also is used along right-ofways, on rangelands, parks, and in aquatic environments. 2,4-D is a chlorine-substituted phenoxyacetic acid herbicide used for postemergence control of annual and perennial broad-leaved weeds in fruits, vegetables, turfs and ornamentals. It is registered with the US Environmental Protection Agency (EPA) for use on a variety of food/feed sites, turf, lawn, aquatic sites, and forestry applications, and as a growth regulator in citrus crops.
What are the applications of Application
2,4-Dichlorophenoxy Acetic Acid is a synthetic auxin supplement
Definition
ChEBI: 2,4-D is a chlorophenoxyacetic acid that is phenoxyacetic acid in which the ring hydrogens at postions 2 and 4 are substituted by chlorines. It has a role as a synthetic auxin, a defoliant, an agrochemical, an EC 1.1.1.25 (shikimate dehydrogenase) inhibitor, an environmental contaminant and a phenoxy herbicide. It is a chlorophenoxyacetic acid and a dichlorobenzene. It is a conjugate acid of a (2,4-dichlorophenoxy)acetate.
Preparation
Two processes are currently used for the production of 2,4-Dichlorophenoxyacetic acid. In the first process, phenol is condensed with chloroacetic acid forming phenoxyacetic acid, which is subsequently chlorinated. In the second process, phenol is chlorinated, generating 2,4-dichlorophenol, which is subsequently condensed with chloroacetic acid. The butyl ester derivative of 2,4-D is produced by the esterification of the acid with butanol in the presence of a ferric chloride catalyst and chlorine (Liu et al., 2013).
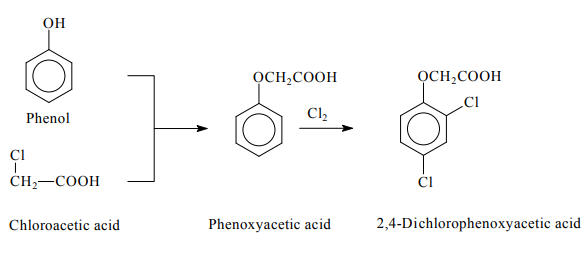
Preparation of 2,4-Dichlorophenoxyacetic acid
Air & Water Reactions
Decomposes rapidly in water.
Reactivity Profile
2,4-Dichlorophenoxyacetic acid is incompatible with strong oxidizers. 2,4-Dichlorophenoxyacetic acid is corrosive to metals.
Health Hazard
The acute toxic symptoms from ingestion,absorption through skin, and inhalation ofits dusts include lethargy, stupor, weakness,incoordination, muscular twitch, nausea, vomiting, gastritis, convulsions, and coma.Death resulted in animals from ventricularfibrillation. In humans, ingestion of 4–6 gof 2,4-D can cause death. The toxic effectsare nausea, vomiting, somnolence, convul-sions, and coma. The oral LD50 value in testanimals varied at 100–500 mg/kg, depend-ing on species, which included dogs, guineapigs, hamsters, rats, mice, and rabbits.
Gorzinski and co-workers (1987) have investigated acute, pharmacokinetic, and subchronic toxicity of 2,4-D and its estersand salts in rats and rabbits. The acutedermal LD50 values in rabbits for theacid, esters, and salts were >2000 mg/kg.Urinary elimination of 2,4-D was saturatedin male rats given oral doses of 50 mg/kg.There was a decrease in body weightgain and increase in kidney weightsin animals, resulting from subchronicdietary doses. A dose-related degenerativechange was observed in the proximalrenal tubules. Repeated subcutaneous dosing(150–250 mg/kg) of 2,4-D butyl esterresulted in accumulation of the compoundin the brain, causing neurobehavioral toxicity(Schulze and Dougherty 1988).
Alexander and associates (1985) determined the 48-hour median lethal con-centration values for 2,4-D to aquatic organ-isms. These values are 25, 325, 290, and358 mg/L for Daphnia magna, fathead min-nows, bluegill, and rainbow trout, respec-tively.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating hydrogen chloride or phosgene gases may form.
Agricultural Uses
Herbicide, Plant growth regulator: 2,4-Dichlorophenoxyacetic acid was introduced as a plant growth-regulator in 1942. 2, 4-D is the most widely used herbicide in the United States and its used in more than 100 countries. It is registered in the United States as a herbicide for control of broadleaf plants and as a plant growth-regulator. There are many forms or derivatives of 2,4-D including esters, amines, and salts. It is used in cultivated agriculture, in pasture and rangeland applications, forest management, home, garden, and to control aquatic vegetation. It may be found in emulsion form, in aqueous solutions (salts), and as a dry compound. The product Agent Orange, made by Monsanto Chemical and used extensively throughout Vietnam, was about 50% 2,4-D. However, the controversies associated with the use of Agent Orange involved a contaminant (dioxin) in the 2,4,5-T component of the defoliant. In 1964 Agent Orange replaced Agent Purple a mixture of the n-butyl esters of 2,4-D and 2,4,5-T plus the isobutyl ester of 2,4,5-T.
Trade name
Hedonal; 2,4-D; Estone; Agrotect; Fernesta; Fernimine; Netagrone; Tributon; Vergemaster; Amoxone; Dicopur; Dormone; Ipaner; Moxone; Phenox; Pielik; Rhodia; Weedone; B-Selektonon.
Potential Exposure
2,4-Dichlorophenoxyacetic acid, was introduced as a plant growth-regulator in 1942. It is registered in the United States as a herbicide for control of broadleaf plants and as a plant growth-regulator. Thus, workers engaged in manufacture, formulation or application are affected, as may be citizens in areas of application. The Vietnam war era defoliant, Agent Orange, was a mixture of 2,4-D and 2,4,5-T.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Carcinogenicity
Collectively, the epidemiological and toxicological data show that 2,4-D is not likely to be carcinogenic in humans unless it is acting through an unknown mechanism that is not evident in animals. According to the calculated RfD and data from exposure studies, the general public should not experience toxic effects from exposure to 2,4-D. Because workers involved in the manufacture or application of 2,4-D may be exposed to levels above the RfD, appropriate protective equipment should be used.
Environmental Fate
2,4-Dichlorophenoxyacetic acid (2,4-d) is rapidly degraded by microbes in soil and water, with a half-life of 3-22 days in different soils. 2,4-d is weakly sorbed by soil with sorption generally increasing with increasing soil organic carbon content. Leaching to groundwater is most likely in coarse-grained sandy soils with low organic content or with very basic soils. In general, little runoff occurs with 2,4-d or its amine salts.
Metabolism
Chemical. 2,4-D and its salts are very stable, but
esters are sensitive to hydrolysis under acidic and basic conditions. In the field, 2,4-D losses due to
photodegradation are minor. 2,4-D is a strong acid and
forms water-soluble salts with amines and alkali metals.
A sequestering agent is included in 2,4-D formulations to
prevent precipitation of Ca2+ andMg2+ salts in hard water.
Plant. 2,4-D detoxification occurs relatively slowly in
plants. There are many possible routes of detoxification,
and these are usually grouped into those reactions that are
consistent with phase I metabolism and those that are consistent
with phase II metabolism. Phase I reactions that
have been observed to occur with 2,4-D include dechlorination,
decarboxylation, hydroxylation, and dealkylation.
Phase II reactions that have been observed to occur
with 2,4-D include conjugation of the side chain to
amino acids, particularly glutamate and aspartate, and glucose conjugation following hydroxylation of the phenoxy
ring. Selectivity differences among broadleaf species
may be accounted for by differences in the rates of 2,4-D
detoxification.
Soil. Microbial degradation in the soil involves cleavage
of the acid side chain, decarboxylation, hydroxylation, and
ring opening.
Storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with 2,4-D,you should be trained on its proper handling and storage.Store in tightly closed containers in a dark, cool, well-ventilated area. Keep away from oxidizers, heat, and sunlight. Aregulated, marked area should be established where thischemical is stored in compliance with OSHA Standard1910.1045.
Shipping
UN3345 Phenoxyacetic acid derivative pesticide, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. A DOT regulated marine pollutant.
Purification Methods
Crystallise 2,4-D from MeOH. It is a plant growth substance, a herbicide and is TOXIC. [Beilstein 6 IV 908.]
Toxicity evaluation
2,4-D is excreted unchanged in the urine of humans and rat. When used under normal conditions, 2,4-D does not appear to produce acute toxic effects on any animal species. The acute oral LD50s of 2,4- D in rat and mouse are 639–764 mg/kg, and 138 mg/kg, respectively.
Incompatibilities
A weak acid, incompatible with bases. Decomposes in sunlight or heat, forming hydrogen chloride and phosgene. Contact with strong oxidizers may cause fire and explosions.
Waste Disposal
Incineration of phenoxys is effective in 1 second @ 982 C, using a straight combustion process or @ 482℃ using catalytic combustion. Over 99% decomposition was reported when small amounts of 2,4-D were burned in a polyethylene bag. See "References" for additional detail. In accordance with 40CFR165, follow (31); recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by follow- ing (100) Package (2) label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 2,4-Dichlorophenoxyacetic acid
| Melting point: | 136-140 °C (lit.) |
| Boiling point: | 160 °C (0.4 mmHg) |
| Density | 1.563 |
| vapor pressure | 0.4 mm Hg ( 160 °C) |
| refractive index | 1.5000 (estimate) |
| Flash point: | 160°C/0.4mm |
| storage temp. | 2-8°C |
| solubility | Soluble in organic solvents (ethanol, acetone, dioxane) |
| form | crystalline |
| pka | pK1:2.64 (25°C) |
| color | off-white to tan |
| PH Range | Acidic |
| Odor Threshold | 3.13 ppm |
| Water Solubility | Slightly soluble. Decomposes. 0.0890 g/100 mL |
| Merck | 14,2796 |
| BRN | 1214242 |
| Henry's Law Constant | 6.72 and 0.84 x 10-5 atm?m3/mol were reported at pH values of 1 and 7, respectively (wetted-wall column, Rice et al.,
1997a) |
| Exposure limits | NIOSH REL: TWA 10 mg/m3, IDLH 100 mg/m3; OSHA PEL: TWA
10 mg/m3; ACGIH TLV: TWA 10 mg/m3. |
| Stability: | Stable, but moisture-sensitive and may be light-sensitive. Incompatible with strong oxidizing agents, corrodes many metals. Decomposes in water. |
| CAS DataBase Reference | 94-75-7(CAS DataBase Reference) |
| NIST Chemistry Reference | (2,4-Dichlorophenoxy)acetic acid(94-75-7) |
| IARC | 2B (Vol. 113) 2018 |
| EPA Substance Registry System | 2,4-D (94-75-7) |
Safety information for 2,4-Dichlorophenoxyacetic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4-Dichlorophenoxyacetic acid
2,4-Dichlorophenoxyacetic acid manufacturer
ALPHA CHEMIKA
Krystal Tech
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 2,4-Dichlorophenoxyacetic Acid CAS 94-75-7View Details
2,4-Dichlorophenoxyacetic Acid CAS 94-75-7View Details
94-75-7 -
 2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
94-75-7 -
 2,4-DICHLOROPHENOXY ACETIC ACID For Synthesis CAS 94-75-7View Details
2,4-DICHLOROPHENOXY ACETIC ACID For Synthesis CAS 94-75-7View Details
94-75-7 -
 2,4-D CAS 94-75-7View Details
2,4-D CAS 94-75-7View Details
94-75-7 -
 2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
94-75-7 -
 2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
2,4-Dichlorophenoxyacetic acid CAS 94-75-7View Details
94-75-7 -
 2,4-D CAS 94-75-7View Details
2,4-D CAS 94-75-7View Details
94-75-7 -
 2,4-Dichlorophenoxy Acetic AcidView Details
2,4-Dichlorophenoxy Acetic AcidView Details
94-75-7
