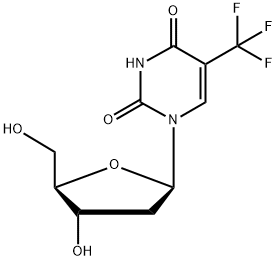
2',3',5'-Tri-O-acetyluridine
Synonym(s):TAU;MAPT;MAPTL;Microtubule-associated protein tau;MTBT1
- CAS NO.:4105-38-8
- Empirical Formula: C15H18N2O9
- Molecular Weight: 370.31
- MDL number: MFCD00023795
- EINECS: 223-881-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-22 20:27:16
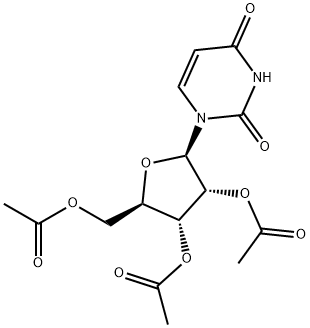
What is 2',3',5'-Tri-O-acetyluridine?
Absorption
Maximum concentrations of uridine in plasma following oral administration are generally achieved within 2 to 3 hours.
Description
Uridine triacetate is an orally available pro-drug of uridine approved by the FDA for the treatment of the rare autosomal recessive disorder called hereditary orotic aciduria. The drug was also approved for treating overdose of two chemotherapies (fluorouracil and capecitabine). Uridine triacetate was developed by Wellstat Therapeutics and licensed to BTG.
Chemical properties
White or almost white crystalline powder
The Uses of 2',3',5'-Tri-O-acetyluridine
2’,3’,5’-Triacetyluridine is used in the treatment of Alzheimer’s disease in mice and provide neuroprotective effects. Shown to also decrease depressive symptoms and increases in brain pH bipolar patients.
Background
Uridine triacetate, formerly known as vistonuridine, is an orally active prodrug of the naturally occurring nucleoside uridine. It is used for the treatment of hereditary orotic aciduria (Xuriden), or for the emergency treatment of fluorouracil or capecitabine overdose or toxicity (Vistogard). It is provided in the prodrug form as uridine triacetate as this form delivers 4- to 6-fold more uridine into the systemic circulation compared to equimolar doses of uridine itself.
When used for the treatment or prevention of toxicity associated with fluorouracil and other antimetabolites, uridine triacetate is utilized for its ability to compete with 5-fluorouracil (5-FU) metabolites for incorporation into the genetic material of non-cancerous cells. It reduces toxicity and cell-death associated with two cytotoxic intermediates: 5-fluoro-2'-deoxyuridine-5'-monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). Normally, FdUMP inhibits thymidylate synthase required for thymidine synthesis and DNA replication and repair while FUTP incorporates into RNA resulting in defective strands. As a result, these metabolites are associated with various unpleasant side effects such as neutropenia, mucositis, diarrhea, and hand–foot syndrome. Like many other neoplastic agents, these side effects limit the doses of 5-FU that can be administered, which also affects the efficacy for treatment. By pre-administering with uridine (as the prodrug uridine triacetate), higher doses of 5-FU can be given allowing for improved efficacy and a reduction in toxic side effects . It can also be used as a rescue therapy if severe side effects present within 96 hours after initiation of therapy.
Uridine triacetate is also used for the treatment of hereditary orotic aciduria, also known as uridine monophosphate synthase deficiency. This rare congenital autosomal recessive disorder of pyrimidine metabolism is caused by a defect in uridine monophosphate synthase (UMPS), a bifunctional enzyme that catalyzes the final two steps of the de novo pyrimidine biosynthetic pathway. As a result of UMPS deficiency, patients experience a systemic deficiency of pyrimidine nucleotides, accounting for most symptoms of the disease. Additionally, orotic acid from the de novo pyrimidine pathway that cannot be converted to UMP is excreted in the urine, accounting for the common name of the disorder, orotic aciduria. Furthermore, orotic acid crystals in the urine can cause episodes of obstructive uropathy. When administered as the prodrug uridine triacetate, uridine can be used by essentially all cells to make uridine nucleotides, which compensates for the genetic deficiency in synthesis in patients with hereditary orotic aciduria. When intracellular uridine nucleotides are restored into the normal range, overproduction of orotic acid is reduced by feedback inhibition, so that urinary excretion of orotic acid is also reduced.
Indications
Marketed as the product Xuriden (FDA), uridine triacetate is indicated for the treatment of hereditary orotic aciduria.
Marketed as the product Vistogard (FDA), uridine triacetate is indicated for the emergency treatment of adult and pediatric patients in the following situations: following a fluorouracil or capecitabine overdose regardless of the presence of symptoms; or who exhibit early-onset, severe or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration.
Definition
ChEBI: Uridine triacetate is an acetate ester that is uracil in which the three hydroxy hydrogens are replaced by acetate group. A prodrug for uridine, it is used for the treatment of hereditary orotic aciduria and for management of fluorouracil toxicity. It has a role as a prodrug, a neuroprotective agent and an orphan drug. It is a member of uridines and an acetate ester.
Synthesis
Commercially available uridine (167) was treated with acetic
anhydride in the presence of catalytic boron trifluoride-etherate,
and the crude product was recrystallized from ethanol to give
uridine triacetate (XX) in 74-78% yield.
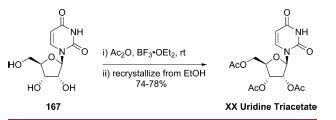
Metabolism
Following oral administration, uridine triacetate is deacetylated by nonspecific esterases present throughout the body, yielding uridine in the circulation.
Properties of 2',3',5'-Tri-O-acetyluridine
| Melting point: | 127.0 to 131.0 °C |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Chloroform (Slightly), Dichloromethane (Slightly), DMSO (Slightly) |
| form | Solid |
| pka | 9.39±0.10(Predicted) |
| color | Off-White |
| InChI | InChI=1S/C15H18N2O9/c1-7(18)23-6-10-12(24-8(2)19)13(25-9(3)20)14(26-10)17-5-4-11(21)16-15(17)22/h4-5,10,12-14H,6H2,1-3H3,(H,16,21,22)/t10-,12-,13-,14-/m1/s1 |
| CAS DataBase Reference | 4105-38-8(CAS DataBase Reference) |
Safety information for 2',3',5'-Tri-O-acetyluridine
Computed Descriptors for 2',3',5'-Tri-O-acetyluridine
| InChIKey | AUFUWRKPQLGTGF-QRMSVCKNSA-N |
| SMILES | C(OC(=O)C)[C@H]1O[C@@H](N2C=CC(=O)NC2=O)[C@H](OC(=O)C)[C@@H]1OC(=O)C |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 2',3',5'-Tri-O-acetyluridine CAS 4105-38-8View Details
2',3',5'-Tri-O-acetyluridine CAS 4105-38-8View Details
4105-38-8 -
 2'',3'',5''-TRIACETYLURIDINE CAS 4105-38-8View Details
2'',3'',5''-TRIACETYLURIDINE CAS 4105-38-8View Details
4105-38-8 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4