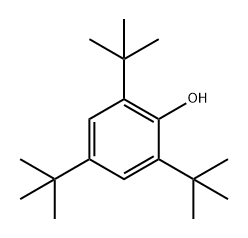
2-tert-Butyl-4,6-dimethylphenol
- CAS NO.:1879-09-0
- Empirical Formula: C12H18O
- Molecular Weight: 178.27
- MDL number: MFCD00002234
- EINECS: 217-533-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
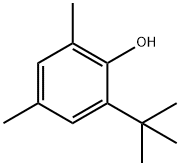
What is 2-tert-Butyl-4,6-dimethylphenol?
The Uses of 2-tert-Butyl-4,6-dimethylphenol
6-tert-Butyl-2,4-xylenol is an antioxidant found used in rocket and jet fuels.
What are the applications of Application
2-tert-butyl-4,6-dimethylphenol is a useful phenol for proteomics research
What are the applications of Application
2-tert-Butyl-4,6-dimethylphenol has been the subject of extensive research due to its diverse biochemistry and organic synthesis applications. This compound serves as a reagent in organic synthesis, facilitating the production of heterocyclic compounds. Additionally, it acts as a catalyst in such syntheses. Its mechanism of action is not yet fully elucidated, but it is believed to involve the activation of diverse signaling pathways. It is used as an antioxidant, e.g., to prevent fuel gumming and as an ultraviolet stabilizer. It is used in jet fuels, gasoline, and avgas.
General Description
A yellow liquid with a phenolic odor. Insoluble in water and about the same density as water. Exposure to skin, eyes or mucous membranes may cause severe burns.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as 2-tert-Butyl-4,6-dimethylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Properties of 2-tert-Butyl-4,6-dimethylphenol
| Melting point: | 22 °C |
| Boiling point: | 249°C |
| Density | 0,917 g/cm3 |
| vapor pressure | 4.2Pa at 25℃ |
| refractive index | 1.5183 |
| Flash point: | 112°C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Insoluble in water |
| pka | 12.00±0.23(Predicted) |
| form | powder to lump to clear liquid |
| color | White or Colorless to Light yellow |
| Water Solubility | 120mg/L at 20℃ |
| FreezingPoint | 20.0 to 24.0 ℃ |
| CAS DataBase Reference | 1879-09-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 6-Tert-butyl-2,4-xylenol(1879-09-0) |
| EPA Substance Registry System | 6-tert-Butyl-2,4-dimethylphenol (1879-09-0) |
Safety information for 2-tert-Butyl-4,6-dimethylphenol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H310:Acute toxicity,dermal H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P391:Collect spillage. Hazardous to the aquatic environment P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for 2-tert-Butyl-4,6-dimethylphenol
| InChIKey | OPLCSTZDXXUYDU-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 6-tert-Butyl-2,4-xylenol CAS 1879-09-0View Details
6-tert-Butyl-2,4-xylenol CAS 1879-09-0View Details
1879-09-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1