2-NITRONAPHTHALENE
- CAS NO.:581-89-5
- Empirical Formula: C10H7NO2
- Molecular Weight: 173.17
- MDL number: MFCD00004055
- EINECS: 209-474-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
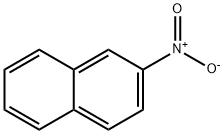
What is 2-NITRONAPHTHALENE?
Chemical properties
yellow to brown crystalline powder or chunks
Definition
ChEBI: A mononitronaphthalene carrying a nitro group at position 2.
Synthesis Reference(s)
Journal of the American Chemical Society, 76, p. 6144, 1954 DOI: 10.1021/ja01652a083
General Description
Yellow crystalline solid. Mp: 76°C; bp: 315°C. Insoluble in water. Very soluble in ethyl alcohol and in diethyl ether. Toxic to aquatic organisms, may cause long-term adverse effects in the environment.
Reactivity Profile
2-NITRONAPHTHALENE is non-flammable but combustible (flash point 160°C). Dust forms explosive mixtures with air. Heating or burning releases toxic and corrosive gases. Incompatible with strong oxidizing agents. Can serve as an oxidizing agent and so react with reducing agents such as hydrides, sulfides and nitrides. Such reactions may be vigorous and may culminate in a detonation. May explode in the presence of strong bases such as sodium or potassium hydroxide even in the presence of water or organic solvents.
Safety Profile
Confirmed carcinogen with experimental tumorigenic data. Moderately toxic by ingestion and intraperitoneal routes. Mutation data reported. A sktn and lung irritant. For explosion and disaster hazards, see NITRATES. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of NOx. See also 1-NITRONAPHTHALENE.
Purification Methods
Distil it in a vacuum and/or crystallise it from aqueous EtOH and sublime in a vacuum. The 1:1 1,3,5-trinitrobenzene complex has m 75.5o (from EtOH). [Beilstein 5 H 555, 5 III 1596, 5 IV 1675.]
Properties of 2-NITRONAPHTHALENE
| Melting point: | 65-73 °C |
| Boiling point: | 303.81°C (rough estimate) |
| Density | 1.279±0.06 g/cm3 (20 ºC 760 Torr) |
| refractive index | 1.5200 (estimate) |
| storage temp. | 2-8°C |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly) |
| form | neat |
| BRN | 2046354 |
| IARC | 3 (Vol. 46) 1989 |
| EPA Substance Registry System | 2-Nitronaphthalene (581-89-5) |
Safety information for 2-NITRONAPHTHALENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H228:Flammable solids H350:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 2-NITRONAPHTHALENE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
