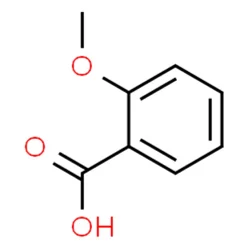
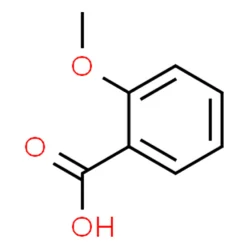
2-Methoxybenzoic acid
Synonym(s):o-Anisic acid;O-Methylsalicylic acid
- CAS NO.:529-75-9
- Empirical Formula: C8H8O3
- Molecular Weight: 152.15
- MDL number: MFCD00002431
- EINECS: 209-447-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-01 20:28:32
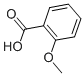
What is 2-Methoxybenzoic acid?
Description
2-Methoxybenzoic acid, also called 2-methoxybenzoate or O-anisic acid, belongs to a group of organic compounds called o-methoxybenzoic acids. These compounds have a methoxy group substituted for the hydrogen atom at position 2 of the benzene ring. In its solid form, 2-methoxybenzoic acid is slightly soluble in water and has weak acidity (indicated by its pKa value). It has been found in various biofluids and s primarily located in the cytoplasm of cells. Additionally, it can be converted into cyprosulfamide. 2-methoxybenzoic acid is present in foods like jew's ear, muscadine grape, sesame, and elliott's blueberry. Therefore, it can potentially be used as a biomarker for the consumption of these food products.
The Uses of 2-Methoxybenzoic acid
2-Methoxybenzoic Acid is used in the preparation of benzohydroxamic acids as potent and selective anti-hepatitis C virus (HCV) agents.
Definition
ChEBI: O-methylsalicylic acid is a methoxybenzoic acid that is the methyl ether of salicylic acid. It has a role as a non-steroidal anti-inflammatory drug and a flavouring agent. It is a conjugate acid of an O-methylsalicylate.
Preparation
The synthesis of 2-methoxybenzoic acid involved dissolving 0.8 kg (20 mol) of sodium hydroxide into 4.7 L of water in a 15 L glass reaction kettle, which was then stirred. The temperature of the reaction kettle was lowered to 0 °C using a low-temperature cycle device. Next, 1.38 kg (10 mol) of salicylic acid was added to the solution and stirred until completely dissolved.
Afterward, 1 L (10.5 mol) of dimethyl sulfate was added to the solution and stirred for 30 minutes at 0 °C. The reaction liquid was then heated to 35 °C for 30 minutes, followed by the sequential addition of 1 L (10.5 mol) of dimethyl sulfate. The temperature was further increased to 45 °C and maintained for 1.0 hour. The mixed solution was then heated to reflux for 2.0 hours.
To adjust the pH of the solution, 40% sodium hydroxide was added until it reached a value of 10. The solution continued to reflux for another 2.0 hours and was naturally cooled to room temperature. Subsequently, hydrochloric acid was used to adjust the pH to 1, resulting in the formation of a significant amount of white precipitate after stirring for 30 minutes.
The final product, 2-methoxybenzoic acid, was obtained by washing and drying the precipitate in an oven, yielding 1.4 kg.
What are the applications of Application
2-Methoxybenzoic acid, also known as 2-methoxybenzoate or O-anisic acid, belongs to the class of organic compounds known as o-methoxybenzoic acids and derivatives. These are benzoic acids in which the hydrogen atom at position 2 of the benzene ring is replaced by a methoxy group. 2-Methoxybenzoic acid has been detected, but not quantified in, several different foods, such as sugar apples (Annona squamosa), cereals and cereal products, fruits, cowpeas (Vigna unguiculata), and other cereal products. This could make 2-methoxybenzoic acid a potential biomarker for the consumption of these foods.
Synthesis Reference(s)
Tetrahedron Letters, 22, p. 1013, 1981 DOI: 10.1016/S0040-4039(01)82853-7
Solubility in water
The water solubility was determined to be 5443.79 mg/L at 30°C.
Properties of 2-Methoxybenzoic acid
| Melting point: | 101-103oC |
| Boiling point: | 182-185 °C (lit.) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), Methanol (Sparingly) |
| form | Solid |
| pka | 4.08(at 25℃) |
| color | White to Off-White |
| JECFA Number | 881 |
| InChI | InChI=1S/C8H8O3/c1-11-7-5-3-2-4-6(7)8(9)10/h2-5H,1H3,(H,9,10) |
Safety information for 2-Methoxybenzoic acid
Computed Descriptors for 2-Methoxybenzoic acid
| InChIKey | ILUJQPXNXACGAN-UHFFFAOYSA-N |
| SMILES | C1(OC)C=CC=CC=1C(=O)O |
2-Methoxybenzoic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 2-Methoxybenzoic acid 99%View Details
2-Methoxybenzoic acid 99%View Details -
 2-Methoxybenzoic acid 98%View Details
2-Methoxybenzoic acid 98%View Details -
 2-METHYOXY BENZOICACID 529-75-9 98% to 99%View Details
2-METHYOXY BENZOICACID 529-75-9 98% to 99%View Details
529-75-9 -
 2 Methoxy Benzoic Acid, 25kg, Grade Standard: Lab GradeView Details
2 Methoxy Benzoic Acid, 25kg, Grade Standard: Lab GradeView Details
529-75-9 -
 2-Methoxy Benzoic Acid, 99%, Grade Standard: IPView Details
2-Methoxy Benzoic Acid, 99%, Grade Standard: IPView Details
529-75-9 -
 2 Methoxybenzoic Acid, 99%, 25KgView Details
2 Methoxybenzoic Acid, 99%, 25KgView Details
579-75-9 -
 Powder 2 Methoxybenzoic AcidView Details
Powder 2 Methoxybenzoic AcidView Details
529-75-9 -
 2-Methoxy Benzoic AcidView Details
2-Methoxy Benzoic AcidView Details
529-75-9
