2-Fluoroethanol
- CAS NO.:371-62-0
- Empirical Formula: C2H5FO
- Molecular Weight: 64.06
- MDL number: MFCD00002828
- EINECS: 206-740-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
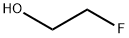
What is 2-Fluoroethanol?
Description
Ethylene fluorohydrin is a colorless liquid.Molecular weight = 64.07; Boiling point = 103.5℃; Flashpoint = 34℃; Vapor pressure = 16 mmHg at 20= C. HazardIdentification (based on NFPA-704 M Rating System):Health 3, Flammability 3, Reactivity 0. Soluble in water.Potential Exposure: Ethylene fluorohydrin is used as arodenticide, insecticide, and acaricide. It is not registered asa pesticide in the United States.
Chemical properties
Ethylene fluorohydrin is a colorless liquid
The Uses of 2-Fluoroethanol
2-Fluoroethanol, is used in the microwave spectra of two isotopic species of 2-fluoroethanol have been investigated. The Gg conformer of 2-fluoroethanol is isolated in low-temperature noble gas matrices. Single-photon IR photolysis of 2-fluoroethanol in solid argon has been investigated.
General Description
A liquid. Used as a rodenticide, insecticide and acaricide. Not registered as a pesticide in the U.S.
Reactivity Profile
2-Fluoroethanol generates very toxic fumes of fluoride that will be emitted in a fire. [EPA, 1998].
Health Hazard
Toxicity rating is the same as for fluoroacetate, super toxic. The probable oral lethal dose in humans is a taste (less than 7 drops) for a 70 kg (150 lb.) person. The chemical is highly toxic when inhaled or absorbed through the skin. Toxicity depends on its oxidation to fluoroacetate by tissue alcohol dehydrogenase.
Fire Hazard
Very toxic fumes of fluoride may be emitted in a fire.
Safety Profile
Poison by inhalation, intraperitoneal, subcutaneous, and intravenous routes. When heated to decomposition it emits very toxic fumes of F-.
Potential Exposure
Ethylene fluorohydrin is used as a rodenticide, insecticide, and acaricide. It is not registered as a pesticide in the U.S.
First aid
Acute poisoning should be treated like poisoningby fluoroacetate. Ethylene fluorohydrin (2-fluoroethanol) islisted among the organic fluorine derivatives of fluoroaceticacid. The emergency procedures for fluoroacetic acid aremove victim to fresh air and call emergency medical care.If not breathing, give artificial respiration. If breathing isdifficult, give oxygen. In case of contact with material,immediately flush skin or eyes with running water for atleast 15 min. Remove and isolate contaminated clothingand shoes at the site. Keep victim quiet and maintain normal body temperature. Effects may be delayed; keep victimunder observation.
storage
(1) Color Code—Red: Flammability Hazard:Store in a flammable liquid storage area or approved cabinet away from ignition sources and corrosive and reactivematerials. (2) Color Code—Blue: Health Hazard/Poison:Store in a secure poison location. Prior to working withthis chemical you should be trained on its proper handlingand storage. Store in tightly closed containers in a cool,well-ventilated area away from oxidizers and reducing agents. Where possible, automatically pump liquidfrom drums or other storage containers to processcontainers.
Shipping
UN3383 Poisonous Toxic by inhalation liquid, flammable, n.o.s. with an LC50 ≤ 200 ml/m3 and saturated vapor concentration ≤ 500 LC50, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid, Technical Name Required, Inhalation Hazard Zone A
Incompatibilities
Ethylene fluorohydrin vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office
Properties of 2-Fluoroethanol
| Melting point: | −26.5 °C(lit.) |
| Boiling point: | 103 °C757 mm Hg(lit.) |
| Density | 1.091 g/mL at 25 °C(lit.) |
| vapor pressure | 16 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 88 °F |
| solubility | Fully miscible. |
| pka | 13.92±0.10(Predicted) |
| form | Oil |
| color | Colourless |
| Sensitive | Air Sensitive |
| BRN | 1730857 |
| CAS DataBase Reference | 371-62-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanol, 2-fluoro-(371-62-0) |
| EPA Substance Registry System | 2-Fluoroethanol (371-62-0) |
Safety information for 2-Fluoroethanol
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H226:Flammable liquids H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P350:IF ON SKIN: Gently wash with plenty of soap and water. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for 2-Fluoroethanol
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 371-62-0 2-Fluoroethanol, 95% 99%View Details
371-62-0 2-Fluoroethanol, 95% 99%View Details
371-62-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1