2-Cyano-6-methoxybenzothiazole
Synonym(s):6-Methoxy-2-benzothiazolecarbonitrile
- CAS NO.:943-03-3
- Empirical Formula: C9H6N2OS
- Molecular Weight: 190.22
- MDL number: MFCD00010537
- EINECS: 231-439-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-29 17:54:30
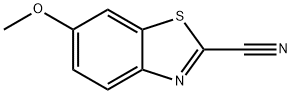
What is 2-Cyano-6-methoxybenzothiazole?
Chemical properties
off-white to light yellow crystalline powder
The Uses of 2-Cyano-6-methoxybenzothiazole
2-Cyano-6-methoxybenzothiazole has been used in the synthesis of:
- firefly luciferin via condensation with cysteine
- luciferin β-glycosides, substrates for novel ultrasensitive enzyme assays
The Uses of 2-Cyano-6-methoxybenzothiazole
Luciferin intermediate.
What are the applications of Application
2-Cyano-6-methoxybenzothiazole is an intermediate of the luciferase substrate luciferin
Synthesis
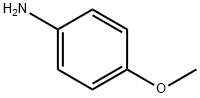
Typical routes to 2-cyano-6-methoxybenzothiazole include the classical Rosenmund-von Braun and Sandmeyer reactions. These methods proceed with low atom economy and require toxic reagents such as KCN, NaCN, Zn(CN)2, or TMSCN, which are also challenging to handle in a large-scale synthesis. Shahmoradi et al. introduced a Cu-catalyzed cyanation of 2-iodo-6-methoxybenzothiazole to synthesize 2-cyano-6-methoxybenzothiazole. K4[Fe(CN)6] was applied as a source of cyanide, and CuI in the presence of N, N N′, N′-tetramethylethylenediamine (TMEDA) was used as part of the catalyst system. 2-Amino-6-methoxybenzothiazole as a starting material was synthesized from p-anisidine as shown below and subsequently converted into 2-iodo-6-methoxybenzothiazole using a simple and efficient one-pot sequential diazotization-iodination method. The one-pot cyanation of 2-iodo-6-methoxybenzothiazole to 2-cyano-6-methoxybenzothiazole was achieved using 0.25 mmol of K4[Fe(CN)6], 0.25 mmol of CuI and 3 mmol of TMEDA in acetonitrile at 160°C. In addition, 1 mmol of mystril trimethyl bromide (MTMAB) was used as a phase transfer agent. The presence of a phase-transfer catalyst is essential for a successful cyanation reaction. Under these conditions, 2-cyano-6-methoxybenzothiazole was produced in a 90% yield[1].
References
[1] Ghasem Shahmoradi, S. Amani. “Synthesis, characterization and computational studies of 2-cyano-6-methoxybenzothiazole as a firefly-luciferin precursor.” Heterocyclic Communications 24 1 (2018): 255–258.
Properties of 2-Cyano-6-methoxybenzothiazole
| Melting point: | 129-131 °C (lit.) |
| Boiling point: | 334.9±34.0 °C(Predicted) |
| Density | 1.2938 (rough estimate) |
| refractive index | 1.6800 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | chloroform: soluble5%, clear, colorless to faintly yellow |
| form | Crystalline Powder |
| pka | -1.49±0.10(Predicted) |
| color | Off-white to light yellow |
| InChI | InChI=1S/C9H6N2OS/c1-12-6-2-3-7-8(4-6)13-9(5-10)11-7/h2-4H,1H3 |
| CAS DataBase Reference | 943-03-3(CAS DataBase Reference) |
Safety information for 2-Cyano-6-methoxybenzothiazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Cyano-6-methoxybenzothiazole
| InChIKey | DEWDWBYQOFXKIH-UHFFFAOYSA-N |
| SMILES | S1C2=CC(OC)=CC=C2N=C1C#N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 943-03-3 2-CYANO-6-METHOXYBENZOTHIAZOLE 98%View Details
943-03-3 2-CYANO-6-METHOXYBENZOTHIAZOLE 98%View Details
943-03-3 -
 943-03-3 98%View Details
943-03-3 98%View Details
943-03-3 -
 2-Cyano-6-methoxybenzothiazole CAS 943-03-3View Details
2-Cyano-6-methoxybenzothiazole CAS 943-03-3View Details
943-03-3 -
 2-Cyano-6-methoxybenzothiazole, 99% CAS 943-03-3View Details
2-Cyano-6-methoxybenzothiazole, 99% CAS 943-03-3View Details
943-03-3 -
 2-Cyano-6-methoxybenzothiazole CAS 943-03-3View Details
2-Cyano-6-methoxybenzothiazole CAS 943-03-3View Details
943-03-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1