2-Chlorobutane
Synonym(s):sec-Butyl chloride;2-Chlorobutane;sec-Butyl chloride
- CAS NO.:78-86-4
- Empirical Formula: C4H9Cl
- Molecular Weight: 92.57
- MDL number: MFCD00000871
- EINECS: 201-151-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
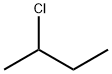
What is 2-Chlorobutane?
Chemical properties
Butyl chloride is a highly flammable, clear, colorless liquid.
The Uses of 2-Chlorobutane
2-Chlorobutane is used as a solvent and intermediate in organic synthesis. Further, it is involved in the preparation of active pharmaceutical ingredients, plasticizer, rubber, resin and surfactants.
Definition
ChEBI: 2-chlorobutane is a chloroalkane that is butane which carries a chloro group at position 2. It derives from a hydride of a butane.
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 2428, 1951 DOI: 10.1021/ja01150a006
Hazard
Flammable, dangerous fire risk.
Flammability and Explosibility
Highly flammable
Safety Profile
2-Chlorobutane is wildly toxic by ingestion, inhalation, and skin contact. Questionable carcinogen with experimental neoplastigenic data. Dangerous fire hazard when exposed to heat, open flame (sparks), or oxidizers. To fight fEe, use water, water spray, fog, mist, dry chemical, alcohol foam. When heated to decomposition it emits toxic fumes of Cl-.
Potential Exposure
Butyl chloride is used as a solvent; as a medicine to control worms, and to make other chemicals
Synthesis
2-Chlorobutane can be synthesized through the addition of hydrochloric acid to 2-butene in the following reaction:

Shipping
UN1127 Chlorobutanes require, Hazard Class: 3; Labels: 3—Flammable liquid
Purification Methods
Purify it in the same way as n-butyl chloride. [Beilstein 1 IV 248.]
Incompatibilities
2-Chlorobutane vapor may form explosive mixture with air. May accumulate static electrical charges, and may cause ignition of its vapors. Water contact slowly forms hydrochloric acid. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alkaline earth, and alkali metals; finely divided metal. Attacks metals in presence of moisture. Attacks some plastics, rubber, or coatings.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of 2-Chlorobutane
| Melting point: | -140 °C (lit.) |
| Boiling point: | 68-70 °C (lit.) |
| Density | 0.873 g/mL at 25 °C (lit.) |
| vapor pressure | 160 hPa (20 °C) |
| refractive index | n |
| Flash point: | 5 °F |
| storage temp. | Store below +30°C. |
| solubility | 1.0g/l |
| form | Liquid |
| color | Clear colorless to slightly yellow |
| PH | 7 (H2O, 20℃)Aqueous solution |
| explosive limit | 2.0-8.8%(V) |
| Water Solubility | immiscible |
| Merck | 14,1561 |
| BRN | 1718770 |
| Dielectric constant | 8.5600000000000005 |
| Stability: | Stable. Flammable - note low flash point. May form explosive mixtures with air. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 78-86-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Butane, 2-chloro-(78-86-4) |
| EPA Substance Registry System | 2-Chlorobutane (78-86-4) |
Safety information for 2-Chlorobutane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for 2-Chlorobutane
2-Chlorobutane manufacturer
Sainor Laboratories Pvt Ltd Unit III
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 78-86-4 SECONDARY - BUTYL CHLORIDE 99%View Details
78-86-4 SECONDARY - BUTYL CHLORIDE 99%View Details
78-86-4 -
 2-Chlorobutane CAS 78-86-4View Details
2-Chlorobutane CAS 78-86-4View Details
78-86-4 -
 2-Chlorobutane CAS 78-86-4View Details
2-Chlorobutane CAS 78-86-4View Details
78-86-4 -
 2-Chlorobutane CAS 78-86-4View Details
2-Chlorobutane CAS 78-86-4View Details
78-86-4 -
 78-86-4 99%View Details
78-86-4 99%View Details
78-86-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
