2-Chloro-2-methylpropane
Synonym(s):tert-Butyl chloride;2-Chloro-2-methylpropane;tert-Butyl chloride
- CAS NO.:507-20-0
- Empirical Formula: C4H9Cl
- Molecular Weight: 92.57
- MDL number: MFCD00000816
- EINECS: 208-066-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-14 12:08:39
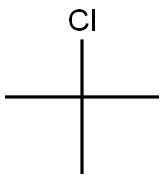
What is 2-Chloro-2-methylpropane?
Chemical properties
tert-Butyl chloride is a colourless liquid. Miscible with alcohol and ether, insoluble in water. It is flammable and releases toxic phosgene when heated. Reacts violently with oxidizing agents. More toxic than 1-chlorobutane. tert-Butyl Chloride has a wide range of applications in the fields of medicine, pesticides, rubber, plastic additives and other chemical industries.
The Uses of 2-Chloro-2-methylpropane
2-Chloro-2-methylpropane is an alkylating agent that is used to functionalize molecules with tert-butyl group.
It serves as an effective chlorinating agent, in combination with the ionic liquid, 1-butyl-3-methylimidazolium bromide for converting alcohols into chlorides.
It can also be used to prepare tert-butyl ethers by treating with corresponding alcohols.
Tert-Butyl chloride Application
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.
Flammability and Explosibility
Flammable
Safety Profile
Questionable carcinogen with experimental neoplastigenic data. Dangerous fire hazard when exposed to heat, flame (sparks), and oxidlzers. To fight fue, use water, spray, fog, alcohol foam, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, ALIPHATIC.
Synthesis
tert-Butyl chloride is prepared from tertbutyl alcohol (tert-butanol) using an acid catalyzed dehydration reaction.
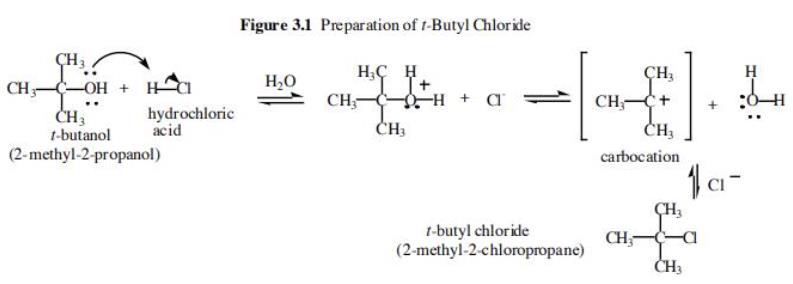
The first step of the overall reaction is an acid-base reaction between the t-butanol and the hydrochloric acid. The t-butanol is a weak base and the hydrochloric acid is a strong acid. The alcoholic oxygen becomes fully protonated and so the equilibrium lies far to the right. In the second step we have the slow loss of water to form a carbocation intermediate. This species is very reactive and is immediately attacked by the chloride ion liberated in the first step to form tert-Butyl chloride.
Tert-Butyl chloride Application
tert-Butyl chloride plays an important role as a starting material to perform nucleophilic substitution reactions in order to prepare alcohol and alkoxides salts. It is used as an alkylating agent for the introduction of tert-butyl group and is also involved in Friedel-Crafts reactions. It is employed as an intermediate for the synthesis of agrochemicals and pharmaceuticals.
Purification Methods
Purification methods commonly used for other alkyl halides lead to decomposition. Some impurities can be removed by photochlorination with a small amount of chlorine prior to use. The liquid is washed with ice water, dried with CaCl2 or CaCl2 + CaO and fractionally distilled. It has been further purified by repeated fractional crystallisation by partial freezing. [Beilstein 1 IV 288.]
Properties of 2-Chloro-2-methylpropane
| Melting point: | -25 °C (lit.) |
| Boiling point: | 51-52 °C (lit.) |
| Density | 0.851 g/mL at 25 °C (lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 4.82 psi ( 20 °C) |
| refractive index | n |
| Flash point: | −10 °F |
| storage temp. | Store at <= 20°C. |
| solubility | soluble in Chloroform |
| form | Liquid |
| color | Clear |
| explosive limit | 1.8-10.1%(V) |
| Water Solubility | SLIGHTLY SOLUBLE |
| Merck | 14,1562 |
| BRN | 1730872 |
| Dielectric constant | 10.949999999999999 |
| Stability: | Stable. Extremely flammable. Note low flash point. Incompatible with strong oxidizing agents. Vapour, being much denser than air, may travel considerable distances to a source of ignition. Hygroscopic. |
| CAS DataBase Reference | 507-20-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Propane, 2-chloro-2-methyl-(507-20-0) |
| EPA Substance Registry System | tert-Butyl chloride (507-20-0) |
Safety information for 2-Chloro-2-methylpropane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for 2-Chloro-2-methylpropane
| InChIKey | NBRKLOOSMBRFMH-UHFFFAOYSA-N |
2-Chloro-2-methylpropane manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 tert-Butyl chloride 507-20-0 98%View Details
tert-Butyl chloride 507-20-0 98%View Details
507-20-0 -
 507-20-0 tert-Butyl chloride 98%View Details
507-20-0 tert-Butyl chloride 98%View Details
507-20-0 -
 tert-Butyl chloride CAS 507-20-0View Details
tert-Butyl chloride CAS 507-20-0View Details
507-20-0 -
 2-Chloro-2-methylpropane CAS 507-20-0View Details
2-Chloro-2-methylpropane CAS 507-20-0View Details
507-20-0 -
 tert-BUTYL CHLORIDE For Synthesis CAS 507-20-0View Details
tert-BUTYL CHLORIDE For Synthesis CAS 507-20-0View Details
507-20-0 -
 2-Chloro-2-methylpropane CAS 507-20-0View Details
2-Chloro-2-methylpropane CAS 507-20-0View Details
507-20-0 -
 2-Chloro-2-methylpropane CAS 507-20-0View Details
2-Chloro-2-methylpropane CAS 507-20-0View Details
507-20-0 -
 TERTIARY BUTYL CHLORIDE(507-20-0)View Details
TERTIARY BUTYL CHLORIDE(507-20-0)View Details
507-20-0
