2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
Synonym(s):2-(3,5-bis(Trifluoromethyl)phenyl)-N,2-dimethyl-N-(6-(4-methylpiperazin-1-yl)-4-o-tolylpyridin-3-yl)propanamide;2-[3,5-bis(Trifluoromethyl)phenyl]-N-[6-(4-methylpiperazin-1-yl)-4-o-tolylpyridin-3-yl]-N-methylisobutyramide;N,a,a-Trimethyl-N-[4-(2-methylphenyl)-6-(4-methyl-1-piperazinyl)-3-pyridinyl]-3,5-bis(trifluoromethyl)benzeneacetamide;/000
- CAS NO.:290297-26-6
- Empirical Formula: C30H32F6N4O
- Molecular Weight: 578.59
- MDL number: MFCD25976831
- EINECS: 1308068-626-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
![2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide Structural](https://img.chemicalbook.in/CAS/GIF/290297-26-6.gif)
What is 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide?
Absorption
Upon oral administration of a single dose of netupitant, netupitant started to be measurable in plasma between 15 minutes and 3 hours after dosing. Plasma concentrations reached Cmax in approximately 5 hours. There was a greater than dose-proportional increase in the systemic exposure with the dose increase from 10 mg to 300 mg and a dose-proportional increase in systemic exposure with a dose increase from 300 mg to 450 mg.
Toxicity
Daily oral administration of netupitant in rats at doses up to 30 mg/kg (1.9 times the human AUC in male rats and 3.7 times the human AUC in female rats at the recommended human dose) had no effects on fertility or reproductive performance.
Description
Netupitant, originally developed by Helsinn Healthcare and later licensed to Eisai, Inc., was approved in the USA in October 2014 for the treatment of chemotherapy-induced nausea and emesis. Akynzeo ® is a fixed-dose combination of the new drug netupitant and the previously-approved 5-HT3 antagonist palonosetron. While palonosetron obtained approval previously for treating nausea and emesis occurring within the first 24 hours (acute phase) after chemotherapy, netupitant provides a synergistic effect with palonosetron, assisting in prevention of nausea and emesis in later stages following chemotherapy (25–120 h after chemotherapy treatment). Several clinical trials showed that this combination of netupitant and palonosetron (Akynzeo ?), in comparison to treatment with palonosetron treatment alone, led to an improved percentage of patients in all phases who did not experience any nausea and emesis after undergoing chemotherapy. Netupitant itself joins the class of selective Neurokinin- 1 (NK1) receptor antagonists which, in addition to their use for treating chemotherapy-induced nausea and emesis, also play an important role as therapies for depression and anxiety.
Description
Netupitant is an insurmountable antagonist of the neurokinin-1 (NK1) receptor (Ki = 0.95 nM in CHO cells expressing the human recombinant receptor). It is selective for human NK1 over human NK2 and NK3 and rat NK1 (Kis = >1,500 nM) and over 50 G protein-coupled receptors, monoamine transporters, and ion channels when used in the nanomolar range. Netupitant decreases the maximal response to substance P-induced contractions in isolated guinea pig ileum with long-lasting effects. It also dose-dependently inhibits the substance P-induced scratching, biting, and licking response in mice when used at doses ranging from 1-10 mg/kg and decreases NK agonist-induced foot tapping in gerbils (ID50s = 1.5 mg/kg, i.p., or 0.5 mg/kg, oral). Formulations containing netupitant have been used in the treatment of chemotherapy-induced nausea and vomiting.
The Uses of 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
Antiemetic.
The Uses of 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
Netupitant is a potent and selective neurokinin-1 receptor (NK1) receptor antagonist. It is achiral and orally active.
Background
Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy. Netupitant is a neurokinin 1 receptor antagonist. The combination drug is marketed by Eisai Inc. and Helsinn Therapeutics (U.S.) Inc. under the brand Akynzeo.
Indications
Netupitant is an antiemitic drug approved by the FDA in October 2014 for use in combination with palonosetron for the prevention of acute and delayed vomiting and nausea associated with cancer chemotherapy including highly emetogenic chemotherapy.
Definition
ChEBI: A monocarboxylic acid amide obtained by formal condensation of the carboxy group of 2-[3,5-bis(trifluoromethyl)phenyl]-2-methylpropanoic acid with the secondary amino group of N-methyl-4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin 3-amine; an antiemetic used in combination with palonosetron hydrochloride (under the trade name Akynzeo) to treat nausea and vomiting in patients undergoing cancer chemotherapy.
Synthesis
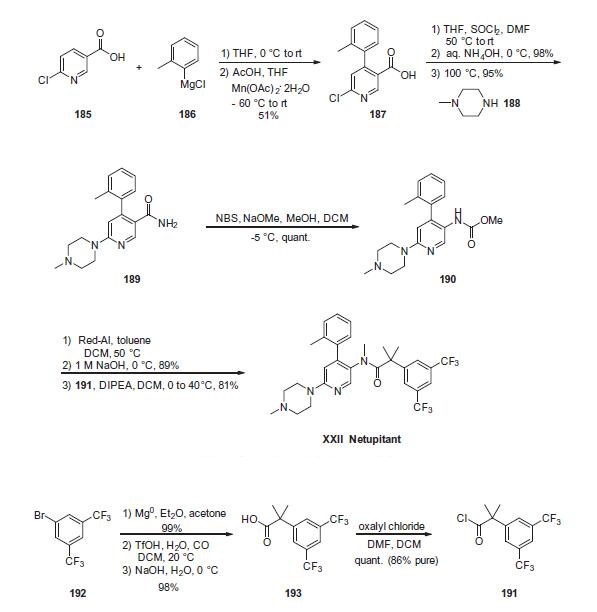
The most likely process-scale synthesis of netupitant begins with 6-chloronicotinic acid (185). From 185, a one-pot 1,4-Grignard addition/oxidation reaction, developed to provide an improved route to NK1 receptor antagonists, was employed for direct installation of the C4-o-tolyl substituent. Using this procedure, treatment of 6-chloronicotinic acid (185) with otolyl magnesium chloride and subsequent oxidation with Mn (OAc)2 in THF/AcOH generated the o-tolyl nicotinic acid intermediate 187 in 51% overall yield. From this intermediate, a one-pot amide formation could be realized in high yield by conversion of the acid to the corresponding acyl chloride and addition of NH4OH (95% yield). Chloride displacement with 1-methyl piperazine under heating conditions provided intermediate 189 in 95% yield. Employing Hoffman reaction conditions originally reported by Senanayake,171 rearrangement of amide 189 with NBS/NaOMe/ MeOH enabled formation of carbamate 190 in quantitative yield. Reduction of the carbamate with Red-Al provided the desired mono-methylated amine. To access the final drug target, acylation of the intermediate methyl amine with 2-(3,5-bis(trifluoromethyl) phenyl)-2-methylpropanoyl chloride (191) provided the final drug netupitant (XXII) in 81% yield. In this case, due to the cost of 193, the acid precursor to 191, and starting materials previously reported for generating 191/193, as well as issues with isolation of pure intermediates on scale, a novel route to 191 and 193 was also developed during this synthesis, beginning with the inexpensive and readily available bromide 192. This 2-step synthesis of 193 includes Grignard reagent formation, quenching with acetone to yield the intermediary tertiary alcohol, and subsequent carbonylation (TfOH, H2O, CO then NaOH/H2O) to provide 2-(3,5-bis(trifluoromethyl)-phenyl)-2- methylpropanoic acid 193. Finally, conversion of acid 193 to the acyl chloride with oxalyl chloride in DCM provided the necessary acyl chloride 191 in quantitative yield (86% purity).
Metabolism
Once absorbed, netupitant is extensively metabolized to form three major metabolites: desmethyl derivative, M1; N-oxide derivative, M2; and OH-methyl derivative, M3. Metabolism is mediated primarily by CYP3A4 and to a lesser extent by CYP2C9 and CYP2D6. Metabolites M1, M2 and M3 were shown to bind to the substance P/neurokinin 1 (NK1) receptor.
Properties of 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
| Melting point: | 156.2-160.0 °C |
| Boiling point: | 597.4±50.0 °C(Predicted) |
| Density | 1.255 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.89±0.38(Predicted) |
| color | White to Off-White |
Safety information for 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide
| InChIKey | WAXQNWCZJDTGBU-UHFFFAOYSA-N |
| SMILES | C(N(C)C1=C(C2C=CC=CC=2C)C=C(N2CCN(C)CC2)N=C1)(=O)C(C1C=C(C(F)(F)F)C=C(C(F)(F)F)C=1)(C)C |
2-[3,5-bis(trifluoromethyl)phenyl]-N,2-dimethyl-N-[4-(2-methylphenyl)-6-(4-methylpiperazin-1-yl)pyridin-3-yl]propanamide manufacturer
New Products
2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Hexanone, 98% Sodium molybdate dihydrate,98% 2,3-Dimethylphenol, 98% 4,4'-Oxydianiline Isoquinoline, 96% 2-Amino-4,6-difluorobenzoic acid, 95% (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Ramipril Sacubitril- Valsartan Boc-his(trt)-OH Fmoc-L-Glu-OtBu Boc-L-Tyr(tBu)-OH N N Dimethylformamide Dimethyl Acetal (Dmf Dma) Semi carbazide Hydrochloride 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] Trans-4-Aminocyclohexanol [4tac] 2-[1-(Mercaptomethyl)Cyclopropyl]Acetic Acid 2-Chloromethyl-4-methyl-quinazolineRelated products of tetrahydrofuran
You may like
-
 290297-26-6 Netupitant 96%View Details
290297-26-6 Netupitant 96%View Details
290297-26-6 -
 Netupitant 98%View Details
Netupitant 98%View Details
290297-26-6 -
 Netupitant 95.00% CAS 290297-26-6View Details
Netupitant 95.00% CAS 290297-26-6View Details
290297-26-6 -
 Netupitant CAS 290297-26-6View Details
Netupitant CAS 290297-26-6View Details
290297-26-6 -
 15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 N-Boc-L-Alanine >98%View Details
15761-38-3 -
 6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 Saccharin Calcium >98%View Details
6485-34-3 -
 Fmoc-Gly-Pro-OH >98%View Details
Fmoc-Gly-Pro-OH >98%View Details
212651-48-4 -
 18568-74-8 >98%View Details
18568-74-8 >98%View Details
18568-74-8