2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
- CAS NO.:104206-65-7
- Empirical Formula: C14H10F3NO5
- Molecular Weight: 329.23
- MDL number: MFCD01752192
- EINECS: 691-056-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
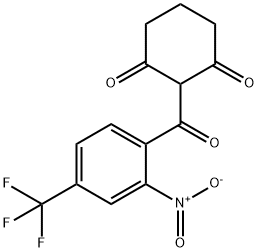
What is 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione?
Absorption
The capsule and liquid formulations are bioequivalent in both the plasma concentration-time curve and maximum plasma concentration (Cmax).
Toxicity
Side effects include elevated plasma levels of this amino acid, hepatic and liver failure.
Description
Nitisinone was originally developed as a pesticide and then launched as an adjunct to dietary restriction of tyrosine and phenylalanine for the treatment of hereditary tyrosinaemia type I. In this inborn error of metabolism, fatal liver disease results either from liver failure during infancy or early childhood or from development of hepatocellular carcinoma during childhood or adolescence. This is caused by accumulation of toxic metabolites due to deficiency of fumarylacetoacetase, the last enzyme of the tyrosine catabolic pathway. Nitisinone, which acts as an inhibitor of the 4-hydroxyphenylpyruvate dioxygenase, prevents the formation of toxic metabolites such as succinylacetoacetate in the liver. Administration of a single dose of nitisinone in mice showed a rapid, significant and persistent inhibition of 4-hydroxyphenylpyruvate dioxygenase. In a murine model of tyrosinaemia type I, administration of nitisinone abolished acute liver failure. Additional dietary tyrosine restriction in the same model on long term follow-up (> 2 years) showed complete correction of liver function tests and succinylacetone levels, and cancer-free survival improvements when compared to historical controls. In healthy volunteers, nitisinone was well tolerated, peak plasma concentrations were rapidly attained following a single dose of 1 mglkg and the half-life time was approximately 54 h. Following the administration of nitisinone (1 mg/kg), the concentrations of tyrosine in plasma increased, were still 8 times those of background at 14 days after dosing, but had returned to background levels within 2 months of the second dose. Elevated tyrosine levels are a potential risk of cornea1 opacities. No treatment related comeal lesions were seen after administration of high dose of nitisinone in mice. In children diagnosed when they were less than 2 months old, when nitisinone treatment was combined with a restricted diet, the four-year survival rate was 88%, compared to 29% from historical data of children treated with restricted diet alone. So, there is some clear evidence that nitisinone treatment associated with restricted diet can reduce the risk of early hepatocellular carcinoma when started before two years of age. On the contrary, in patients with late start of nitisinone treatment there is a considerable risk of liver malignancy. Even if 10% of patients have not clinically responded to nitisinone, studies have shown that oral nitisinone treatment plus dietary restriction has greatly improved the survival of patients and reduced the need of liver transplantation during early childhood.
Chemical properties
Light Brown Solid
Originator
AstraZeneca (UK)
The Uses of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
Nitisinone is a herbicidal triketone that inhibits 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme involved in plastoquinone biosynthesis in plants and in tyrosine catabolism in mammals. It is u sed in treatment of inherited tyrosinemia type I.
The Uses of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
Nitisinone is a herbicidal triketone that inhibits 4-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme involved in plastoquinone biosynthesis in plants and in tyrosine catabolism in mammals. It is used in treatment of inherited tyrosinemia type I.
Indications
Used as an adjunct to dietary restriction of tyrosine and phenylalanine in the treatment of hereditary tyrosinemia type 1.
Background
Nitisinone is a synthetic reversible inhibitor of 4-hydroxyphenylpyruvate dioxygenase. It is used in the treatment of hereditary tyrosinemia type 1. It is sold under the brand name Orfadin.
What are the applications of Application
Nitisinone is an herbicidil triketone that inhibits 4-hydroxyphenylpyruvate dioxygenase (HPPD)
Definition
ChEBI: A cyclohexanone that is cyclohexane-1,3-dione substituted at position 2 by a 2-nitro-4-(trifluoromethyl)benzoyl group. It is used in the treatment of hereditary tyrosinemia type 1.
brand name
. Orfadin (Swedish Orphan).
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards. Nitisinone is a medication, effectively used for the treatment and maintainence of alkaptonuria, an autosomal recessive disease. Its mode of action involves the inhibition of second enzyme in the tyrosine pathway, 4-hydroxyphenylpyruvate dioxygenase that produces HGA from 4-hydroxyphenylpyruvate.
Biochem/physiol Actions
Nitisinone is a competitive inhibitor that reversibly inhibits 4-Hydroxyphenylpyruvate oxidase (dioxygenase). Nitisinone is used in the treatment of hereditary tyrosinemia type 1, where it blocks the degradation of tyrosine into harmful substances.
Pharmacokinetics
Hereditary tyrosinemia type 1 occurs due to a deficiency in fumarylacetoacetase (FAH), the final enzyme in the tyrosine catabolic pathway. Nitisinone inhibits catabolism of tyrosine by preventing the catabolic intermediates. In patients with HT-1, these catabolic intermediates are converted to the toxic metabolites succinylacetone and succinylacetoacetate, which are responsible for the observed liver and kidney toxicity. Succinylacetone can also inhibit the porphyrin synthesis pathway leading to the accumulation of 5-aminolevulinate, a neurotoxin responsible for the porphyric crises characteristic of HT-1.
Metabolism
Not Available
Properties of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
| Melting point: | 129-131°C |
| Boiling point: | 486.2±45.0 °C(Predicted) |
| Density | 1.484±0.06 g/cm3(Predicted) |
| RTECS | GV0766666 |
| storage temp. | -20°C |
| solubility | DMSO: ≥5mg/mL |
| pka | 3.13±0.50(Predicted) |
| form | Solid |
| color | white to brown |
| EPA Substance Registry System | 1,3-Cyclohexanedione, 2-[2-nitro-4-(trifluoromethyl)benzoyl]- (104206-65-7) |
Safety information for 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
Computed Descriptors for 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
| InChIKey | OUBCNLGXQFSTLU-UHFFFAOYSA-N |
Abamectin manufacturer
Biophore India Pharmaceuticals Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 104206-65-7 Nitisinone 98%View Details
104206-65-7 Nitisinone 98%View Details
104206-65-7 -
 104206-65-7 98%View Details
104206-65-7 98%View Details
104206-65-7 -
 Nitisinone 99%View Details
Nitisinone 99%View Details
104206-65-7 -
 Nitisinone 104206-65-7 98%View Details
Nitisinone 104206-65-7 98%View Details
104206-65-7 -
 104206-65-7 99%View Details
104206-65-7 99%View Details
104206-65-7 -
 Nitisinone 95% CAS 104206-65-7View Details
Nitisinone 95% CAS 104206-65-7View Details
104206-65-7 -
 Nitisinone CAS 104206-65-7View Details
Nitisinone CAS 104206-65-7View Details
104206-65-7 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4