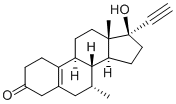
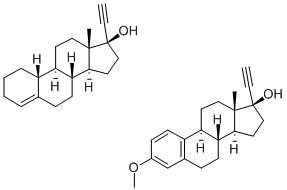
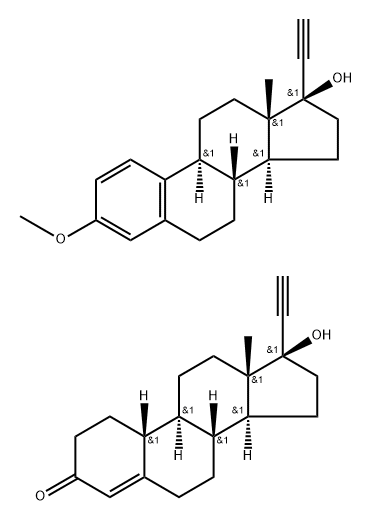
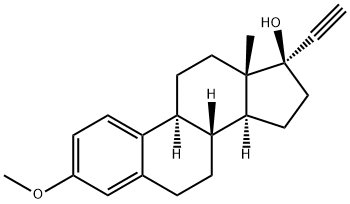
17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
Synonym(s):(17α)-3-Methoxy-19-norpregna-1,3,5(10)-trien-20-yn-17-ol;17α-Ethynyl-1,3,5(10)-estratriene-3,17β-diol 3-methyl ether;17α-Ethynylestradiol 3-methyl ether;3-Methoxy-17α-ethynylestradiol;NSC 84032
- CAS NO.:72-33-3
- Empirical Formula: C21H26O2
- Molecular Weight: 310.43
- MDL number: MFCD00003689
- EINECS: 200-777-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
What is 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether?
Chemical properties
Crystalline Solid
Originator
Enovid ,Searle ,US ,1957
The Uses of 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
Mestranol is an orally active estrogenic steroid. It was the estrogen used in many of the first oral contraceptives
The Uses of 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
antiemetic
The Uses of 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
Pharmacological estrogen molecule
Background
The 3-methyl ether of ethinyl estradiol. It must be demethylated to be biologically active. It is used as the estrogen component of many combination ORAL contraceptives.
Indications
Mestranol was used as one of the first oral contraceptives.
What are the applications of Application
Mestranol is an orally active estrogenic steroid
Definition
ChEBI: A terminal acetylenic compound that is (17alpha)-17-ethynylestra-1(10),2,4-triene substituted by a methoxy group at position 3 and a hydroxy group at position 17.
Manufacturing Process
A stirred solution of 120 parts of 3-methoxy-δ1,3,5-estratrien-17-one in 2,600 parts of anhydrous toluene and 4,300 parts of anhydrous ether is saturated with a slow stream of acetylene. In the course of 30 minutes there is added a solution of 120 parts of potassium tert-amylate in 2,800 parts of anhydrous tert-pentanol. The passage of acetylene and stirring are continued for an additional 5 hours after which the reaction mixture is washed 5 times with 3,000-part portions of saturated ammonium chloride solution and then with water. It is then dried over anhydrous sodium sulfate and concentrated to dryness under vacuum. The residue is recrystallized from methanol. The 3- methoxy-17-ethynyl-δ1,3,5 estratrien-17-ol thus obtained melts at about 143° to 146°C. A further recrystallization from acetone yields crystals melting at about 150° to 151°C.
Therapeutic Function
Estrogen
Safety Profile
Confirmed carcinogen with experimental. neoplastigenic, tumorigenic, and teratogenic data. Moderately toxic by subcutaneous route. Human reproductive effects by ingestion: changes in ovaries and fallopian tubes, ferthty effects. Experimental reproductive effects. Mutation data reported. An FDA proprietary drug. A steroid used in oral contraceptives. When heated to decomposition it emits acrid smoke and irritating fumes.
Metabolism
Properties of 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
| Melting point: | 153-155 °C(lit.) |
| Boiling point: | 390.58°C (rough estimate) |
| alpha | +2~+8°(D/20℃)(c=1, 1,4-dioxane) |
| Density | 1.0865 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile: 1 mg/ml; Ethanol: 1 mg/ml; Methanol: 1 mg/ml |
| form | Solid |
| pka | 13.10±0.40(Predicted) |
| form | neat |
| color | White to off-white |
| optical activity | [α]15/D +3°, c = 2 in dioxane |
| Merck | 5917 |
| BRN | 2625905 |
| CAS DataBase Reference | 72-33-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Mestranol(72-33-3) |
| EPA Substance Registry System | Mestranol (72-33-3) |
Safety information for 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether
17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol 3-methyl ether manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Mestranol CAS 72-33-3View Details
Mestranol CAS 72-33-3View Details
72-33-3 -
 Mestranol CAS 72-33-3View Details
Mestranol CAS 72-33-3View Details
72-33-3 -
 Mestranol CAS 72-33-3View Details
Mestranol CAS 72-33-3View Details
72-33-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6