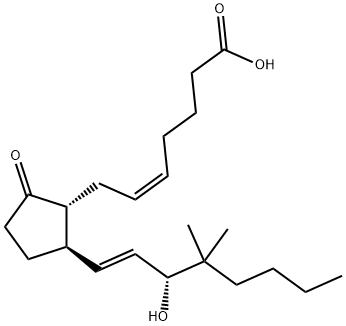
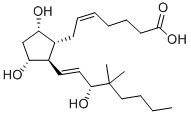
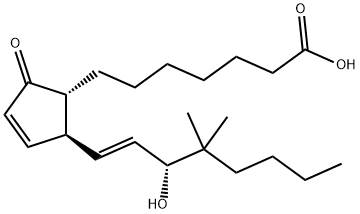
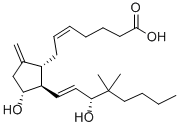
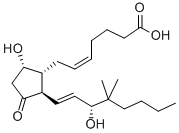
16,16-DIMETHYL PROSTAGLANDIN E2
Synonym(s):16,16-Dimethyl-PGE2
- CAS NO.:39746-25-3
- Empirical Formula: C22H36O5
- Molecular Weight: 380.52
- MDL number: MFCD00151141
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
What is 16,16-DIMETHYL PROSTAGLANDIN E2?
Description
16,16-dimethyl PGE2 is a competitive inhibitor of 15-
What are the applications of Application
16,16-Dimethyl-prostaglandin E2 is an antisecretory, antiulcer PGE2 analog that is resistant to metabolism
Definition
ChEBI: 16,16-dimethylprostaglandin E2 is a prostanoid that is prostaglandin E2 in which both of the hydrogens at position 16 have been replaced by methyl groups. A synthetic analogue of prostaglandin E2, it is a potent inhibitor of pancreatic function and growth of experimental tumors. It also protects the gastric mucosa, prevents ulceration, and promotes the healing of peptic ulcers. It has a role as a radiation protective agent, an anti-ulcer drug and a gastrointestinal drug. It is a prostanoid, a monocarboxylic acid, a secondary allylic alcohol and a member of cyclopentanones.
in vitro
dmpge2 was reported to cause an increase in runx11/cmyb1 hscs, while hscs were inhibited by indomethacin treatment in 90% of embryos. moreover, dmpge2 had minimal effects on the vasculature, while indomethacin altered the intersomitic vessels slightly. imaged by confocal microscopy, red-labelled hscs and endothelium embryos showed significantly increased hscs following dmpge2 exposure [1].
in vivo
in a heterotopic model of rat allograft rejection, dmpge2 could delay the rejection onset, but all animals developed severe rejection and died subsequently. treatment of animals with low-dose csa in combination with dmpge2 led to a delay in the onset as well as a reduction in the intensity of allograft rejection. in addition, a statistical relationship between procoagulant activity levels and the time of onset of rejection was observed [1].
storage
Store at -80°C
References
[1] north te,goessling w,walkley cr,lengerke c,kopani kr,lord am,weber gj,bowman tv,jang ih,grosser t,fitzgerald ga,daley gq,orkin sh,zon li. prostaglandin e2 regulates vertebrate haematopoietic stem cell homeostasis. nature.2007 jun 21;447(7147):1007-11.
[2] koh ih,kim pc,chung sw,waddell t,wong py,gorczynski r,levy ga,cohen z. the effects of 16, 16 dimethyl prostaglandin e2 therapy alone and in combination with low-dose cyclosporine on rat small intestinal transplantation. transplantation.1992 oct;54(4):592-8.
Properties of 16,16-DIMETHYL PROSTAGLANDIN E2
| Flash point: | -13℃ |
| storage temp. | -20°C |
| solubility | Soluble in methyl acetate |
| form | methyl acetate solution |
| color | Colorless to light yellow |
Safety information for 16,16-DIMETHYL PROSTAGLANDIN E2
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 16,16-DIMETHYL PROSTAGLANDIN E2
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4