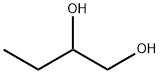
1,3-Butanediol
Synonym(s):(±)-1,3-Butanediol;1,3-Butylene glycol
- CAS NO.:107-88-0
- Empirical Formula: C4H10O2
- Molecular Weight: 90.12
- MDL number: MFCD00004554
- EINECS: 203-529-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08

What is 1,3-Butanediol?
Chemical properties
colourless liquid
Chemical properties
Butylene glycol occurs as a clear, colorless, viscous liquid with a sweet flavor and bitter aftertaste.
Chemical properties
1,3-Butanediol is a colorless oily liquid. Sp.Gr. 1.00. B.P. 207℃. Miscible with water, soluble in alcohol and many perfume and flavor materials, but poorly soluble in hydrocarbons (terpenes, etc.). 1,3-Butylene glycol has a sweet flavor with bitter aftertaste and is odorless when pure.
The Uses of 1,3-Butanediol
1,3-Butanediol is used in the synthesis of colchicine derivatives as anticancer agents. Also used in the synthesis of dual peroxisome proliferator-activated gamma and delta agonists acting as euglycem ic agents in the treatment of diabetes.
The Uses of 1,3-Butanediol
Its most extensive use is as an intermediate in the manufacture of polyester plasticisers and other chemical products. It finds some use as a solvent and humectant, a useful chemical intermediate. It has extensive application in the manufacture of structural materials for boats, custom mouldings, and sheets and boards for construction applications. 1,3-Butanediol imparts resistance to weathering plus flexibility and impact resistance. It is also used in the manufacture of saturated polyesters for polyurethane coatings, where the glycol imparts greater flexibility to the polyester molecule. 1,3-Butanediol is currently used in many personal care products.
The Uses of 1,3-Butanediol
(^+)-1,3-Butanediol acts as a co-monomer in the production of polyurethane and polyester resins. It is used as a humectant (to prevent loss of moisture) in cosmetics, especially in hair sprays and setting lotions. It is used in surfactants, inks, solvents for natural and synthetic flavorings. It is involved in the synthesis of dual peroxisome proliferator-activated gamma and delta agonists acting as euglycemic agents, which is used in the treatment of diabetes.
What are the applications of Application
(±)-1,3-Butanediol is a building block for proteomics research
Definition
ChEBI: A butanediol compound having two hydroxy groups in the 1- and 3-positions.
Production Methods
Butylene glycol is prepared by catalytic hydrogenation of aldol using Raney nickel.
Preparation
From formaldehyde and propylene via pressure and a catalyst.
Aroma threshold values
Detection: 70 to 100 ppm
General Description
(±)-1,3-Butanediol (BD) is a 1,3-diol. Its vapor pressure upto 270kPa, liquid-phase densities over a temperature range, two-phase (liquid + vapor) heat capacities, critical temperature and critical density have been determined. The obtained data was employed to derive various thermophysical properties.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Butylene glycol is used as a solvent and cosolvent for injectables.
It is used in topical ointments, creams, and lotions, and it is also
used as a vehicle in transdermal patches. Butylene glycol is a good
solvent for many pharmaceuticals, especially estrogenic substances.
In an oil-in-water emulsion, butylene glycol exerts its best
antimicrobial effects at ~8% concentration. Higher concentrations
above 16.7% are required to inhibit fungal growth.
Contact allergens
This dihydric alcohol is used for its humectant and preservative potentiator properties in cosmetics, topical medicaments and polyurethane, polyester, cellophane, and cigarettes. It has similar properties, but is less irritant than propylene glycol. Contact allergies seem to be rare.
Safety Profile
Mdly toxic by ingestion and subcutaneous routes. A skin and eye irritant. See also ETHYLENE GLYCOL. Experimental reproductive effects. Combustible when exposed to heat or flame. Incompatible with oxidizing materials. To fight fire, use foam, alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Butylene glycol is used in a wide variety of cosmetic formulations
and is generally regarded as a relatively nontoxic material. It is
mildly toxic by oral and subcutaneous routes.
In topical preparations, butylene glycol is regarded as minimally
irritant. Butylene glycol can cause allergic contact dermatitis, with
local sensitivity reported in patch tests. Some local irritation
is produced on eye contact.
LD50 (guinea pig, oral): 11.0 g/kg
LD50 (mouse, oral): 12.98 g/kg
LD50 (rat, oral): 18.61 g/kg
LD50 (rat, SC): 20.0 g/kg
Carcinogenicity
There were no tumors found in the 2-year feeding studies on dogs and rats . Thus, it appears that 1,3-butanediol is not carcinogenic.
Metabolism
Not Available
Storage
Butylene glycol is hygroscopic and should be stored in a well-closed container in a cool, dry, well-ventilated place. When heated to decomposition, butylene glycol emits acrid smoke and irritating fumes.
Incompatibilities
Butylene glycol is incompatible with oxidizing reagents.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (transdermal patches). Included in licensed medicines in the UK (topical gel patches/medicated plasters).
Properties of 1,3-Butanediol
| Melting point: | -54 °C |
| Boiling point: | 203-204 °C(lit.) |
| Density | 1.005 g/mL at 25 °C(lit.) |
| vapor density | 3.1 (20 °C, vs air) |
| vapor pressure | 0.06 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 250 °F |
| storage temp. | Store below +30°C. |
| solubility | >500g/lMiscible |
| form | Liquid |
| pka | 14.83±0.20(Predicted) |
| Specific Gravity | 1.004 – 1.007 |
| color | Clear colorless to yellow, may discolor to brown on storage |
| Odor | odorless |
| PH Range | 6 - 7 at 20 °C |
| PH | 6.1 (500g/l, H2O, 20℃) |
| explosive limit | 1.9-12.6%(V) |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,1567 |
| BRN | 1731276 |
| Stability: | Stable. Flammable. Hygroscopic - protect from air and moisture. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 107-88-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3-Butanediol(107-88-0) |
| EPA Substance Registry System | 1,3-Butylene glycol (107-88-0) |
Safety information for 1,3-Butanediol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 1,3-Butanediol
1,3-Butanediol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 1,3-Butanediol 98%View Details
1,3-Butanediol 98%View Details -
 1, 3 Butylene Glycol CASView Details
1, 3 Butylene Glycol CASView Details -
 1,3 Butylene Glycol CASView Details
1,3 Butylene Glycol CASView Details -
 (±)-1,3-Butanediol CAS 107-88-0View Details
(±)-1,3-Butanediol CAS 107-88-0View Details
107-88-0 -
 1 3 Butylene GlycolView Details
1 3 Butylene GlycolView Details
107-88-0 -
 1 3 Butylene GlycolView Details
1 3 Butylene GlycolView Details
107-88-0 -
 1 3 Butylene Glycol, LooseView Details
1 3 Butylene Glycol, LooseView Details
107-88-0 -
 1, 3 Butylene GlycolView Details
1, 3 Butylene GlycolView Details
107-88-0
