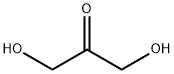
2-Butyne-1,4-diol
Synonym(s):1,4-Dimethoxyacetylene;2-Butyne-1,4-diol
- CAS NO.:110-65-6
- Empirical Formula: C4H6O2
- Molecular Weight: 86.09
- MDL number: MFCD00002915
- EINECS: 203-788-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is 2-Butyne-1,4-diol?
Chemical properties
yellow solid. Soluble in water, acidic solution, ethanol and acetone, slightly soluble in chloroform, insoluble in benzene and ether.
The Uses of 2-Butyne-1,4-diol
2-Butyne-1,4-diol is used as a precursor to prepare 1,4-butanediol, 2-butene-1,4-diol and mucochloric acid. It is also used in textile additives, corrosion inhibitors, plasticizers, synthetic resins and polyurethanes. It is an important raw material of vitamin B6. Further, it is used for brightening, preserving and inhibiting nickel plating. In addition, it is used in biological studies for nematocidal activity.
Definition
ChEBI: 2-Butyne-1,4-diol is a butynediol that is but-2-yne substituted by hydroxy groups at positions 1 and 4. It is used to produce butanedioland butenediol, in metal plating andpickling baths, and in making the carbamateherbicide Barban (Carbyne).
What are the applications of Application
Basic brightener in nickel electroplating baths, also an important intermediate for organic synthesis; corrosion inhibitor; defoliant ; polymerization accelerator; stabilizer for chlorinated hydrocarbons; cosolvent for paint and varnish removal.
General Description
1,4-butynediol appears as white to light-brown solid or brownish-yellow aqueous solution. Solid sinks and mixes with water. (USCG, 1999)
Air & Water Reactions
Water soluble.
Reactivity Profile
Pure 2-Butyne-1,4-diol is non-explosvie. Small amounts of certain impurities-alkali hydroxides, alkaline earth hydroxides, halides-may cause explosive decomposition upon distillation. 2-Butyne-1,4-diol should not be treated with basic catalysts in the absence of a solvent at room temperature, and its stability is less with elevated temperatures. In strong acids, contamination with mercury salts can also result in violent decomposition [NFPA 491M 1991].
Hazard
Toxic by ingestion. May explode on contamination with mercury salts, strong acids, and alkaline earth hydroxides and halides at high temperatures. May cause dermatitis.
Health Hazard
2-Butyne-1,4-diol exhibits moderate to hightoxicity in test animals. It is about 10 timesmore toxic than are the saturated C4 diols.The oral LD50 value in rats is 0.125 mL/kg.It causes irritation to the skin.
Fire Hazard
Noncombustible solid, flash point (open cup) 152°C (305.6°F). It is stable at room temperature. But the dry compound explodes in the presence of certain heavy metal salts, such as mercuric chloride. Heating with alkaline solution may result in an explosion.
Safety Profile
A poison by ingestion. A skin sensitizer upon long or repeated contact. Moderately explosive. When heated to decomposition it emits acrid smoke and fumes and may explode. Explosive reaction with traces of alkaltes, alkali earth hydroxides, halide salts, strong acids, mercury salts + strong acids. See also ACETYLENE COMTOUNDS.
Synthesis
2-butyne-1,4-diol is carried out by reaction under pressure of acetylene and an aqueous solution of formaldehyde, catalyzed by copper acetylide.
Storage
2-Butyne-1,4-diol can be stored in steel,aluminum, nickel, glass, epoxy, and phenolicliner containers. Rubber hose may be usedfor transfer. Avoid contact with heavy metalsalt contaminants.
Purification Methods
Crystallise the diol from EtOAc. [Beilstein 1 IV 2687.]
Properties of 2-Butyne-1,4-diol
| Melting point: | 54 °C |
| Boiling point: | 238 °C(lit.) |
| Density | 1.2 |
| vapor pressure | <0.1 mm Hg ( 55 °C) |
| refractive index | 1.4804 |
| Flash point: | 306 °F |
| storage temp. | Store below +30°C. |
| solubility | 3740g/l |
| form | Crystalline Platelets or Flakes |
| pka | 12.72±0.10(Predicted) |
| color | slightly brown |
| PH | 4-7.5 (100g/l, H2O, 23℃) |
| Water Solubility | 3740 g/L (20 ºC) |
| BRN | 1071237 |
| Exposure limits | ACGIH: TWA 0.1 ppm; STEL 0.3 ppm OSHA: TWA 0.75 ppm; STEL 2 ppm NIOSH: IDLH 20 ppm; TWA 0.016 ppm; Ceiling 0.1 ppm |
| Stability: | Stable. Highly flammable solid. Incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, strong acids, strong bases. |
| CAS DataBase Reference | 110-65-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butyne-1,4-diol(110-65-6) |
| EPA Substance Registry System | 2-Butyne-1,4-diol (110-65-6) |
Safety information for 2-Butyne-1,4-diol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Butyne-1,4-diol
2-Butyne-1,4-diol manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 110-65-6 98%View Details
110-65-6 98%View Details
110-65-6 -
 2-Butyne-1,4-diol CAS 110-65-6View Details
2-Butyne-1,4-diol CAS 110-65-6View Details
110-65-6 -
 2-Butyne-1,4-diol CAS 110-65-6View Details
2-Butyne-1,4-diol CAS 110-65-6View Details
110-65-6 -
 2-Butyne-1,4-diol CAS 110-65-6View Details
2-Butyne-1,4-diol CAS 110-65-6View Details
110-65-6 -
 1,4 Butynediol CAS Number : 110-65-6, 99%View Details
1,4 Butynediol CAS Number : 110-65-6, 99%View Details
110-65-6 -
 1,4-ButynediolView Details
1,4-ButynediolView Details
110-65-6 -
 1, 4 ButynediolView Details
1, 4 ButynediolView Details
110-65-6 -
 2-Butyne-1,4-DiolView Details
2-Butyne-1,4-DiolView Details
110-65-6
