1,3-Dimethylamylamine
Synonym(s):1,3-Dimethylpentanamine;1,3-Dimethylpentylamin;4-Methyl-2-hexanamine;Methylhexanamine;NSC 1106
- CAS NO.:105-41-9
- Empirical Formula: C7H17N
- Molecular Weight: 115.22
- MDL number: MFCD00025613
- EINECS: 203-296-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-29 17:45:46
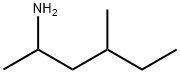
What is 1,3-Dimethylamylamine?
Description
1,3-dimethylamylamine or methylexaneamine (DMAA) is a synthetic pharmaceutical patented in the 1940s as a nasal decongestant which can be used as a recreational stimulant. Alleged to occur in nature,DMAA has become a widely used ingredient in sports food supplements, despite its status as a doping agent and concerns over its safety. The most popular brands containing DMAA include Jack3d? and OxyElite Pro?, both marketed by USP Labs and both detained by the FDA in July 2013 (however, some can still be found on Amazon and other online retail stores).
Description
1,3-Dimethylamylamine is also known as methylhexan(e)amine and many trade names. In 1944 and 1945, Eli Lilly was awarded US patents for its synthesis; and beginning in 1971, it was marketed by Lilly as a nasal decongestant. It is also sold as a dietary supplement and stimulant. In 2010, the World Anti-Doping Agency added it to the list of substances prohibited for use by athletes.
Chemical properties
White or almost white powder
Originator
Forthane,Lilly,US,1948
The Uses of 1,3-Dimethylamylamine
DMAA (1,3-dimethylamylamine) is an amphetamine derivative that has been widely used in sports supplements sold in the United States. Also known as methylhexanamine or geranium extract, DMAA is often touted as a "natural" stimulant, with many claimed functional uses including a body-building aid, an athletic performance enhancer, and a weight-loss aid. Although DMAA at one time was approved as a drug for nasal decongestion, no medical use of DMAA is recognized today. FDA is not aware of any reliable science indicating that DMAA exists naturally in plants.
What are the applications of Application
1,3-Dimethylamylamine (1,3-DMAA) is a neural stimulant with a structure similar to ephedrine and adrenaline that has been used as a pre-workout stimulant. It is useful in compositions, for example as dietary supplements, and for appetite suppression. Methylhexaneamine is a botanical ingredient in dietary supplements. Methylhexaneamine and caffeine has been combined in attempts to improve exercise performance and related variables.
Definition
ChEBI: Methylhexaneamine is an alkylamine.
Definition
Compared to the commonly available stimulant caffeine, DMAA has a longer t1/2, in this case 8.4 h vs. 5.4 hr for caffeine, as well as a shorter lag time of 0.14 h vs. 0.37 h for caffeine. Previous reports have indicated that DMAA is absorbed over 4–12 hours.
Manufacturing Process
One molecular equivalent of 4-methylhexanone-2 is reacted with slightly more than one molecular equivalent of hydroxylamine. Desirably, the hydroxylamine is prepared in the presence of the 4-methylhexanone-2 by reacting the hydrochloride or sulfate or other salt of the hydroxylamine with a suitable base, such as sodium carbonate or sodium hydroxide. Desirably, the reaction mixture is agitated for a few hours to insure the conversion of the 4- methylhexanone-2 to 4-methylhexanone-2 oxime.
The resulting 4-methylhexanone-2 oxime separates and is dried by any
suitable means, such as with a dehydrating agent, for example, sodium
sulfate or magnesium sulfate. After drying, 4-methylhexanone-2 oxime is
reduced with hydrogen by means of a catalyst, such as Raney nickel, or by
reaction of sodium and a primary alcohol, such as ethanol. The resulting 2-
amino-4-methylhexane may be purified by distillation, as described in US
Patent 2,350,318.
115 g (1 mol) of 2-amino-4-methylhexane and 9 g (0.5 mol) of water are
placed in a tared 500 cc 3-necked flask which is equipped with a mechanical
stirrer, a thermometer, and a gas delivery tube. The flask is surrounded by a
cooling bath of ice and water. Dry carbon dioxide gas is introduced into the
solution through the gas delivery tube, with constant stirring, until the
increase in weight is approximately 22 g (0.5 mol). The temperature during
this addition is maintained between 20° and 30°C. A viscous liquid results,
and consists essentially of 2-amino-4-methylhexane carbonate. This also
dissociates very slowly at room temperature to the free amine, carbon
dioxide, and water; and is effective as an inhalant, according to US Patent
2,386,273.
Therapeutic Function
Nasal decongestant
Pharmacokinetics
Oral Clearance (CL) values (CL/F; F represents oral bioavailability) were found to be relatively low (20±5.0 L/hr), whereas oral Volume of distribution (V/F) was relatively high (236±38.0 L). A 25 mg single oral dose resulted in a maximum plasma concentration (Cmax) of 76.5 ng/mL, which occurred approximately 4 hours post-ingestion. Cmaxvalues were approximately 15-30 times lower than those reported in case studies linking 1,3-Dimethylamylamine (DMAA) intake with severe adverse events, suggesting that the reported adverse events were associated with a DMAA intake much higher than the 25 mg dose in our study [9,10]. Additionally, the potential impact of drug-drug interactions should be considered when interpreting DMAA adverse event reports. Because DMAA PK data suggest that a significant fraction of an orally administered DMAA dose is metabolized, concomitant administration of common ingredients that are also metabolized (e.g., caffeine), may lead to exaggerated DMAA concentrations by inhibiting DMAA clearance.
Side Effects
Taking 1,3-Dimethylamylamine (DMAA) can raise blood pressure and lead to cardiovascular problems ranging from shortness of breath and tightening in the chest to heart attack. It may also cause stroke, a condition called lactic acidosis, heart attack, liver injury, and death.
Properties of 1,3-Dimethylamylamine
| Melting point: | -19°C (estimate) |
| Boiling point: | bp760 130-135° |
| Density | 0.7620-0.7655 |
| refractive index | n20/D1.417-1.419 |
| Flash point: | 43℃ |
| form | neat |
| pka | pKa 10.54(H2O,t =24±1,I=0.002) (Uncertain) |
| InChI | InChI=1S/C7H17N/c1-4-6(2)5-7(3)8/h6-7H,4-5,8H2,1-3H3 |
| CAS DataBase Reference | 105-41-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Hexanamine, 4-methyl-(105-41-9) |
Safety information for 1,3-Dimethylamylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,3-Dimethylamylamine
| InChIKey | YAHRDLICUYEDAU-UHFFFAOYSA-N |
| SMILES | CC(N)CC(C)CC |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
