1,2,3,5-Tetrachlorobenzene
- CAS NO.:634-90-2
- Empirical Formula: C6H2Cl4
- Molecular Weight: 215.89
- MDL number: MFCD00000543
- EINECS: 211-217-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
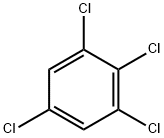
What is 1,2,3,5-Tetrachlorobenzene ?
The Uses of 1,2,3,5-Tetrachlorobenzene
1,2,3,5-Tetrachlorobenzene is used in the synthesis of several organic compounds including that of polyarylated benzenes by multiple Suzuki-Miyaura reactions. It was used as part of study which investigated the antimicrobial activity of tetra substituted benzene derivatives which suggested antimicrobial activity tended to decrease with increasing size of halogen substituents.
Definition
ChEBI: A tetrachlorobenzene carrying chloro groups at positions 1, 2, 3 and 5.
General Description
White crystals or off-white solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Simple aromatic halogenated organic compounds, such as 1,2,3,5-Tetrachlorobenzene , are very unreactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. 1,2,3,5-Tetrachlorobenzene may react with oxidizers. .
Health Hazard
ACUTE/CHRONIC HAZARDS: 1,2,3,5-Tetrachlorobenzene may cause irritation on contact.
Fire Hazard
1,2,3,5-Tetrachlorobenzene is probably combustible.
Environmental Fate
Biological. A mixed culture of soil bacteria or a Pseudomonas sp. transformed 1,2,3,5-tetrachlorobenzene
to 2,3,4,6-tetrachlorophenol (Ballschiter and Scholz, 1980). The half-life of 1,2,3,5-
tetrachlorobenzene in an anaerobic enrichment culture was 1.8 d (Beurskens et al., 1993).
In an enrichment culture derived from a contaminated site in Bayou d’Inde, LA, 1,2,3,5-
tetrachlorobenzene underwent reductive dechlorination to 1,2,4- and 1,3,5-trichlorobenzene at
relative molar yields of 7 and 93%, respectively. The maximum dechlorination rate, based on the
recommended Michaelis-Menten model, was 94 nM/d (Pavlostathis and Prytula, 2000).
Photolytic. Irradiation (λ ≥285 nm) of 1,2,3,5-tetrachlorobenzene (1.1–1.2 mM/L) in an
acetonitrile-water mixture containing acetone (0.553 mM/L) as a sensitizer gave the following
products (% yield): 1,2,3-trichlorobenzene (5.3), 1,2,4-trichlorobenzene (4.9), 1,3,5-trichlorobenzene
(49.3), 1,3-dichlorobenzene (1.8), 2,3,4,4′,5,5′,6-heptachlorobiphenyl (1.41), 2,2′,3,4,4′,6,6′-
heptachlorobiphenyl (1.10), 2,2′,3,3′,4,5′,6-heptachlorobiphenyl (4.50), four hexachlorobiphenyls
(4.69), one pentachlorobiphenyl (0.64), trichloroacetophenone, 1-(trichlorophenyl)-2-propanone,
and (trichlorophenyl)acetonitrile (Choudhry and Hutzinger, 1984). Without acetone, the identified
photolysis products (% yield) included 1,2,3-trichlorobenzene (trace), 1,2,4-trichlorobenzene
(24.3), 1,3,5-trichlorobenzene (11.7), 1,3-dichlorobenzene (0.5), 1,4-dichlorobenzene (3.3),
pentachlorobenzene (1.43), 1,2,3,4-tetrachlorobenzene (5.99), two heptachlorobiphenyls (1.40),
two hexachlorobiphenyls (<0.01), and one pentachlorobiphenyl (0.75) (Choudhry and Hutzinger,
1984).
Purification Methods
Crystallise it from EtOH. [Beilstein 5 II 157, 5 III 551, 5 IV 668.]
Properties of 1,2,3,5-Tetrachlorobenzene
| Melting point: | 54.5°C |
| Boiling point: | 246°C |
| Density | 1.5578 (estimate) |
| vapor pressure | 0.07 at 25 °C (extrapolated, Mackay et al., 1982) |
| refractive index | 1.5348 (estimate) |
| Flash point: | 113 °C |
| storage temp. | APPROX 4°C
|
| solubility | Soluble in alcohol, benzene, ether, and ligroin (Weast, 1986) |
| form | neat |
| color | White to Almost white |
| Water Solubility | 4.016mg/L(25 ºC) |
| BRN | 1618864 |
| Henry's Law Constant | 58.0 at 25 °C (gas stripping-GC, Shiu and Mackay, 1997) |
| CAS DataBase Reference | 634-90-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2,3,5-tetrachloro-(634-90-2) |
| EPA Substance Registry System | 1,2,3,5-Tetrachlorobenzene (634-90-2) |
Safety information for 1,2,3,5-Tetrachlorobenzene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 1,2,3,5-Tetrachlorobenzene
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
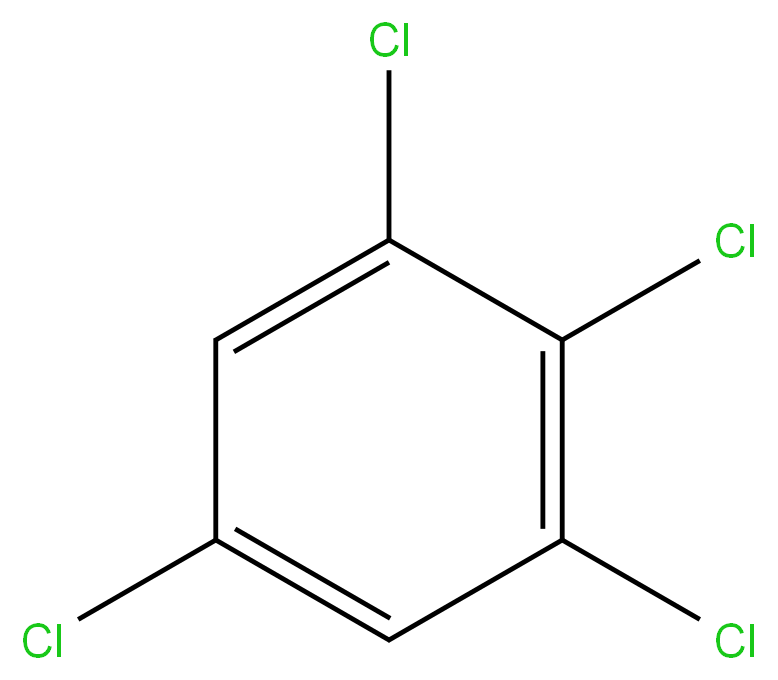 634-90-2 1,2,3,5-TETRA CHLORO BENZENE 98%View Details
634-90-2 1,2,3,5-TETRA CHLORO BENZENE 98%View Details
634-90-2 -
 1,2,3,5-Tetrachlorobenzene CAS 634-90-2View Details
1,2,3,5-Tetrachlorobenzene CAS 634-90-2View Details
634-90-2 -
 1,2,3,5-Tetrachlorobenzene CAS 634-90-2View Details
1,2,3,5-Tetrachlorobenzene CAS 634-90-2View Details
634-90-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1