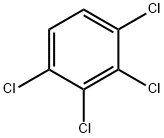
1,2,3,4-Tetrachlorobenzene
- CAS NO.:634-66-2
- Empirical Formula: C6H2Cl4
- Molecular Weight: 215.89
- MDL number: MFCD00000538
- EINECS: 211-214-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is 1,2,3,4-Tetrachlorobenzene?
Chemical properties
WHITE CRYSTALLINE SOLID
The Uses of 1,2,3,4-Tetrachlorobenzene
1,2,3,4-tetrachlorobenzene was used as a model compound to determine the chlorobenzenes (CBs) in soil samples. It was also used for rapid dechlorination of 1,2,3,4-tetrachlorodibenzo-p-dioxin (1,2,3,4-TeCDD).
The Uses of 1,2,3,4-Tetrachlorobenzene
Component of dielectric fluids, synthesis.
Definition
ChEBI: A tetrachlorobenzene carrying chloro groups at positions 1, 2 , 3 and 4.
General Description
White to off-white crystals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Simple aromatic halogenated organic compounds, such as 1,2,3,4-Tetrachlorobenzene, are very unreactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides.
Health Hazard
ACUTE/CHRONIC HAZARDS: 1,2,3,4-Tetrachlorobenzene may cause irritation of the skin.
Fire Hazard
1,2,3,4-Tetrachlorobenzene is probably combustible.
Safety Profile
Moderately toxic by ingestion. An experimental teratogen. Experimental reproductive effects. Irritant. Combustible liquid. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC.
Environmental Fate
Biological. A mixed culture of soil bacteria or a Pseudomonas sp. transformed 1,2,3,4-
tetrachlorobenzene to 2,3,4,5-tetrachlorophenol (Ballschiter and Scholz, 1980). After incubation in
sewage sludge for 32 d under anaerobic conditions, 1,2,3,4-tetrachlorobenzene did not biodegrade
(Kirk et al., 1989). The half-life of 1,2,3,4-tetrachlorobenzene in an anaerobic enrichment culture
was 26.4 h (Beurskens et al., 1993). Potrawfke et al. (1998) reported that a pure culture of
Pseudomonas chlororaphis RW71 mineralized 1,2,3,4-tetrachlorobenzene as a sole source of
carbon and energy. Intermediate biodegradation products identified were tetrachlorocatechol,
tetrachloromuconic acid, 2,3,5-trichlorodienelactone, 2,3,5-trichloro-4-hydroxymuconic acid.
In an enrichment culture derived from a contaminated site in Bayou d’Inde, LA, 1,2,4,5-
tetrachlorobenzene underwent reductive dechlorination yielding 1,2,4-trichlorobenzene. The
maximum dechlorination rate, based on the recommended Michaelis-Menten model, was 208
nM/d (Pavlostathis and Prytula, 2000).
Photolytic. Irradiation (λ ≥285 nm) of 1,2,3,4-tetrachlorobenzene (1.1–1.2 mM/L) in an
acetonitrile-water mixture containing acetone (0.553 mM/L) as a sensitizer gave the following
products (% yield): 1,2,3-trichlorobenzene (9.2), 1,2,4-trichlorobenzene (32.6), 1,3-dichlorobenzene
(5.2), 1,4-dichlorobenzene (1.5), 2,2′,3,3′,4,4′,5-heptachlorobiphenyl (2.52), 2,2′,3,3′,4,5,6′-
heptachlorobiphenyl (1.22), 10 hexachlorobiphenyls (3.50), five pentachlorobiphenyls (0.87),
dichlorophenyl cyanide, two trichloroacetophenones, trichlorocyanophenol, (trichlorophenyl)
acetonitriles, and 1-(trichlorophenyl)-2-propanone (Choudhry and Hutzinger, 1984). Without
acetone, the identified photolysis products (% yield) included 1,2,3-trichlorobenzene (7.8), 1,2,4-
trichlorobenzene (26.8), 1,2-dichlorobenzene (0.5), 1,3-dichlorobenzene (0.7), 1,4-dichloro-benzene
(30.4), 1,2,3,5-tetrachlorobenzene (2.26), 1,2,4,5-tetrachlorobenzene (0.72), 2,2′,3,3′,4,4′,5-
heptachlorobiphenyl (<0.01), and 2,2′,3,3′,4,5,6′-heptachlorobiphenyl (<0.01) (Choudhry and
Hutzinger, 1984). The sunlight irradiation of 1,2,3,4-tetrachlorobenzene (20 g) in a 100-mL
borosilicate glass-stoppered Erlenmeyer flask for 56 d yielded 4,280 ppm heptachlorobiphenyl
(Uyeta et al., 1976).
Solubility in water
Soluble in acetic acid, ether, ligroin (Weast, 1986), and very soluble in many chlorinated solvents including chloroform, carbon tetrachloride, etc.
Purification Methods
Crystallise it from EtOH. [Beilstein 5 H 204, 5 II 156, 5 III 550, 5 IV 667.]
Properties of 1,2,3,4-Tetrachlorobenzene
| Melting point: | 44 °C |
| Boiling point: | 254 °C761 mm Hg(lit.) |
| Density | 1,73 g/cm3 |
| vapor pressure | 1.5 (extrapolated from vapor pressures determined at higher temperatures, Tesconi andYalkowsky, 1998) |
| refractive index | 1.5348 (estimate) |
| Flash point: | >230 °F |
| storage temp. | APPROX 4°C
|
| solubility | 0.0028g/l |
| form | neat |
| color | White crystals or needles |
| Water Solubility | 5.92mg/L(25 ºC) |
| BRN | 1910025 |
| Henry's Law Constant | 3.6 at 10 °C (Koelmans et al., 1999) |
| Dielectric constant | 3.2000000000000002 |
| CAS DataBase Reference | 634-66-2(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2,3,4-Tetrachlorobenzene (634-66-2) |
Safety information for 1,2,3,4-Tetrachlorobenzene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 1,2,3,4-Tetrachlorobenzene
1,2,3,4-Tetrachlorobenzene manufacturer
Cns Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 1,2,3,4-Tetrachlorobenzene 634-66-2 99%View Details
1,2,3,4-Tetrachlorobenzene 634-66-2 99%View Details
634-66-2 -
 634-66-2 1,2,3,4 - Tetrachloro benzene 98%View Details
634-66-2 1,2,3,4 - Tetrachloro benzene 98%View Details
634-66-2 -
 1,2,3,4-Tetrachlorobenzene 98%View Details
1,2,3,4-Tetrachlorobenzene 98%View Details -
 1,2,3,4-Tetrachlorobenzene 98%View Details
1,2,3,4-Tetrachlorobenzene 98%View Details
634-66-2 -
 1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
634-66-2 -
 1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
634-66-2 -
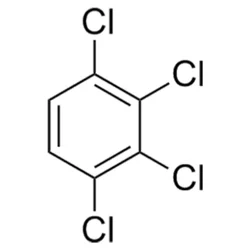 1,2,3,4-TetrachlorobenzeneView Details
1,2,3,4-TetrachlorobenzeneView Details
634-66-2 -
 1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
1,2,3,4-Tetrachlorobenzene CAS 634-66-2View Details
634-66-2
