1,2-DIPALMITOYL-SN-GLYCEROL
- CAS NO.:30334-71-5
- Empirical Formula: C35H68O5
- Molecular Weight: 568.91
- MDL number: MFCD00067471
- EINECS: 250-131-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
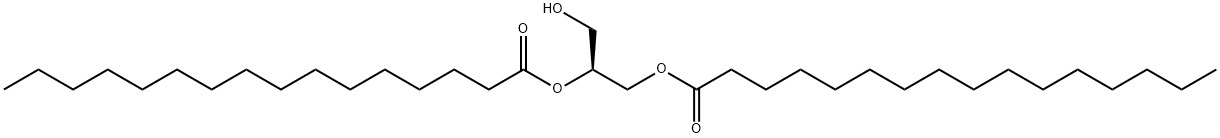
What is 1,2-DIPALMITOYL-SN-GLYCEROL?
The Uses of 1,2-DIPALMITOYL-SN-GLYCEROL
1,2-Dipalmitoyl-sn-glycerol (1,2-DPG) is an analog of the protein kinase C (PKC)-activating second messenger diacylglycerol. 1,2-Dipalmitoyl-sn-glycerol is a weak activator of PKC.
The Uses of 1,2-DIPALMITOYL-SN-GLYCEROL
16:0 DG has been used to spike brain samples for mass spectrometric analysis. It may be used as diacyl-glycerol source in diacylglycerol O-acyltransferase 1 (DGAT1) enzyme assay in human leukemic?K562 cells and in Golgi-like liposomes reconstitution.
What are the applications of Application
1,2-Dipalmitoyl-sn-glycerol is a DAG analog and weak PKC activator
Definition
ChEBI: A 1,2-diacyl-sn-glycerol in which both acyl groups are specified as palmitoyl (hexadecanoyl).
General Description
In biochemical signaling, diacylglycerol (DAG) functions as a second messenger signaling lipid, and is a product of the hydrolysis of the phospholipid PIP2 (phosphatidylinositolbisphosphate) by the enzyme phospholipase C (PLC) (a membrane-bound enzyme) that, through the same reaction, produces inositol trisphosphate (IP3). Although inositol trisphosphate (IP3) diffuses into the cytosol, DAG remains within the plasma membrane due to its hydrophobic properties. IP3 stimulates the release of calcium ions from the smooth endoplasmic reticulum, whereas DAG is a physiological activator of protein kinase C (PKC). The production of DAG in the membrane facilitates translocation of PKC from the cytosol to the plasma membrane.
Diacylglycerol mimicks the effects of the tumor-promoting compounds phorbol esters.
Biochem/physiol Actions
16:0 DG (1,2-dipalmitoyl-sn-glycerol) fails to prevent the late fission events in Golgi membrane. Supplementation of 16:0 DG promotes bacterial growth by preventing sporangia.
Purification Methods
Crystallise S(-)-1,2-dipalmitin from chloroform/pet ether (b 40-60o) ~1:1.5. [S(-)-isomer: Baer & Kates J Am Chem Soc 72 942 1950, Hanahan & Vercamer J Am Chem Soc 7 6 1804 1954, R(+)-isomer: Tattrie et al. Arch Biochem 78 319 1958, Beilstein 2 IV 1173.] The racemate [40290-32-2] is polymorphic with different IR spectra. When crystallised from hexane, or other solvents, the higher melting form with m 71.5-72.5o is obtained. The melt then solidifies to give the lower melting -form with m 49.7-50o. The -form has m 61o (65-66o is also reported). When the lower melting forms are kept at their melting temperatures for a while, they are converted to the higher melting form. [Howe & Malkin J Chem Soc 2663 1951, Baer & Kates J Am Chem Soc 72 942 1950, Beilstein 2 IV 1173.]
Properties of 1,2-DIPALMITOYL-SN-GLYCEROL
| Melting point: | 66-69 °C |
| Boiling point: | 620.8±22.0 °C(Predicted) |
| Density | 0.930±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMF: 20 mg/ml; DMSO: 5 mg/ml; Ethanol: 30 mg/ml; PBS (pH 7.2): .25 mg/ml |
| form | A crystalline solid |
| pka | 13.69±0.10(Predicted) |
| color | White to off-white |
| BRN | 1730209 |
Safety information for 1,2-DIPALMITOYL-SN-GLYCEROL
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,2-DIPALMITOYL-SN-GLYCEROL
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1,2-DIOCTADECANOYL-SN-GLYCERO-3-PHOSPHO-RAC-[1-GLYCEROL] AMMONIUM SALT](https://img.chemicalbook.in/CAS/GIF/108347-80-4.gif)
You may like
-
 1,2-Dipalmitoyl-sn-glycerol CAS 30334-71-5View Details
1,2-Dipalmitoyl-sn-glycerol CAS 30334-71-5View Details
30334-71-5 -
 16:0 DG CAS 30334-71-5View Details
16:0 DG CAS 30334-71-5View Details
30334-71-5 -
 16:0 DG CAS 30334-71-5View Details
16:0 DG CAS 30334-71-5View Details
30334-71-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1