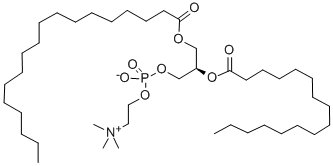
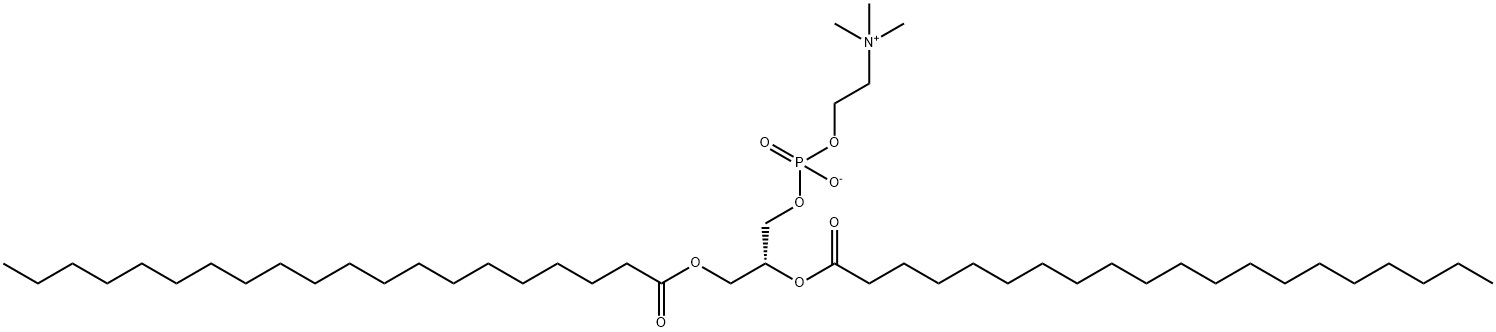
1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
Synonym(s):PC;DPPC;PC(16:0/16:0);(7R)-4-Hydroxy-N,N,N-trimethyl-10-oxo-7-[(1-oxohexadecyl)oxy]- 3,5,9-trioxa-4-phosphapentacosan-1-aminium 4-oxide, inner salt;L -α-Phosphatidylcholine, dipalmitoyl
- CAS NO.:63-89-8
- Empirical Formula: C40H80NO8P
- Molecular Weight: 734.04
- MDL number: MFCD00036903
- EINECS: 200-567-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
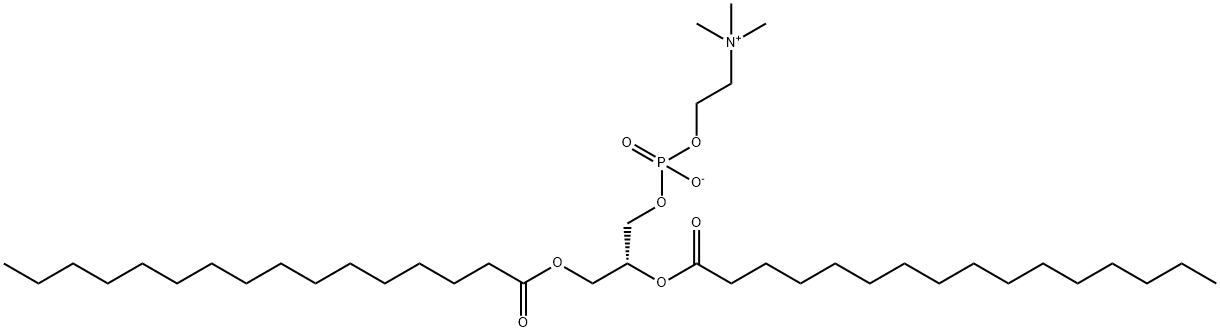
What is 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE?
Absorption
The absorption is done directly in the alveolus into the lung tissue. As the lung surfactant is distributed in the bronchi, bronchioles and alveoli, its highest concentration is at the alveolar air-fluid interface where it remains as a monolayer.
Toxicity
In clinical trials, there are reports of pulmonary hemorrhage when colfosceril palmitate is administered in infants with a weight of fewer than 700 grams at birth. Another potential risk of the use of colfosceril palmitate is the formation of mucus plugging in the endotracheal tube which can be prevented by performing suction prior to dosing.
Description
DPPC, also known as 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine, is a phospholipid containing the 16 carbon fatty acid/palmitic acid (16:0) at the sn-1 and sn-2 positions. It is commonly used in the generation of micelles, liposomes, and other types of artificial membranes. Due to the medium size of fatty acid chain, DLPC is used to form thinner membranes or lipid bilayers.
Chemical properties
white powder
The Uses of 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
1,2-dipalmitoyl-sn-glycero-3-phosphocholine is a major phospholipid constituent and primary surface tension lowering component of natural lung surfactant. Colfosceril Palmitate is used as pulmonary surfactant and diagnostic aid (fetal lung maturity).
The Uses of 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
16:0 PC (DPPC) is suitable for use:
- in vesicle preparation
- as the main phospholipid component for all ultrasound contrast agents (UCA) formulations
- as a component for microbubble preparation
- in the preparation of lipid bilayers
The Uses of 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
16:0 PC (DPPC) has been used in the generation of thymoquinone (TQ) liposomes, gold nanorods and calcium chloride encapsulated liposomes and bilayers for biophysical studies.
Background
Colfosceril palmitate is a synthetic pulmonary surfactant administered in infants with respiratory distress syndrome. It was part of the first generation of commercially available artificial surfactants. It was developed by Burroughs Wellcome and it was FDA approved on August 6, 1990. Nowadays colfosceril palmitate is under the state of canceled post-marketing.
Indications
Colfosceril palmitate is indicated for the treatment of respiratory distress syndrome (RDS) in premature infants. The official label is referred as a intratracheal suspension for prophylactic treatment of infants of less than 1350 grams of birth weight under risk of developing RDS, or in infants with birth weight greater than 1350 grams with pulmonary immaturity, or as rescue treatment of infants that already developed RDS. The central feature of RDS is a surfactant deficiency due to lung immaturity. This lung condition is more frequently presented due to risk factors like prematurity, delayed lung maturation caused by maternal diabetes or male gender, or surfactant dysfuntion due to perinatal asphyxia, pulmonary infection or delivery without labor.
What are the applications of Application
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine is a phosphoglyceride that has been used for preparation of liposomal monolayers/bilayers
Definition
ChEBI: A phosphatidylcholine 32:0 in which the 1- and 2-acyl groups are specified as hexadecanoyl (palmitoyl). A synthetic phospholipid used in liposomes and lipid bilayers to study biological membranes. It is also a major constituent of pulmonary surfactants.
General Description
Phosphatidylcholine (PC) is a strong bilayer-forming lipid. It the most common phospholipid in mammalian membranes. It is also an important component of the mucosal layer of the colon.
Biochem/physiol Actions
Phosphatidylcholine (PC) functions as a surfactant within the mucus to form a hydrophobic surface to inhibit bacterial penetrance. It is used to treat fat embolism. Phosphatidylcholine lowers the levels of cholesterol and triglycerides.
Pharmacokinetics
Colfosceril palmitate has shown to significantly reduce the risk of pneumothoraces, pulmonary interstitial emphysema and mortality. Unlike naturals surfactants, colfosceril palmitate reduces the risk of bronchopulmonary dysplasia, intraventricular hemorrhage and patent ductus arteriosus. In clinical placebo-controlled trials, there was a significant reduction in the number of deaths attributed to hyaline membrane disease, the incidence of pulmonary air leaks, oxygen requirements and mean airway pressure. Some reports have indicated a lack of therapeutic effect due to the absence of surfactant protein.
Metabolism
Colfosceril palmitate is catabolized and reutilized for further synthesis and secretion in lung tissues.
Purification Methods
It has three crystalline forms 1, 2 and ' which change at 60-70o and at 229o, respectively. In order to obtain a fine powder, ~2 g are dissolved in CHCl3 (15mL) and pet ether (b 35-60o) is added; the solution is evaporated to dryness in vacuo at <20o and then dried at 0.1mm over CaCl2. [Baer & Maurukas J Am Chem Soc 74 158 1952, Baer & Kates J Biol Chem 185 615 1950, Beilstein 4 IV 1463.]
Properties of 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Melting point: | 229-229.5 °C |
| alpha | D23 +6.6° (c = 4.2 in 1:1 chloroform-methanol) |
| storage temp. | -20°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White to Off-White |
| Merck | 14,2477 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 63-89-8(CAS DataBase Reference) |
Safety information for 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 1,2-DIPALMITOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| InChIKey | KILNVBDSWZSGLL-KXQOOQHDSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
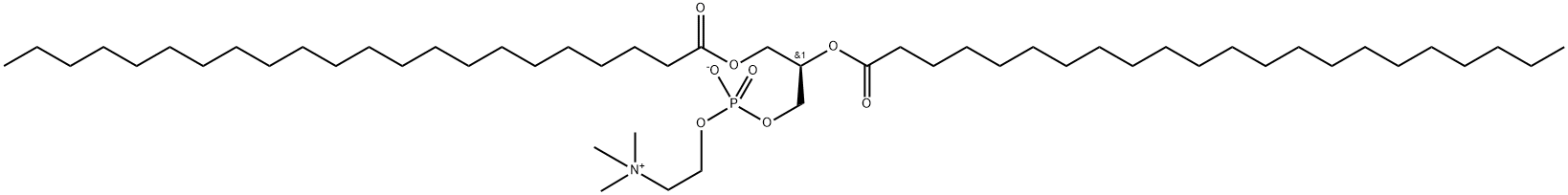


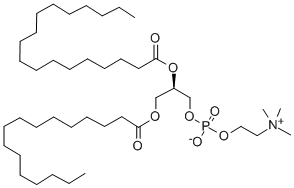
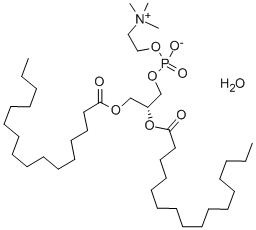
![PHOSPHATIDYLCHOLINE, L-ALPHA-DIPALMITOYL, [CHOLINE-METHYL-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB9684731.gif)
You may like
-
 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
63-89-8 -
 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
63-89-8 -
 Colfosceril palmitate 98.00% CAS 63-89-8View Details
Colfosceril palmitate 98.00% CAS 63-89-8View Details
63-89-8 -
 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
63-89-8 -
 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine CAS 63-89-8View Details
63-89-8 -
 16:0 PC (DPPC) CAS 63-89-8View Details
16:0 PC (DPPC) CAS 63-89-8View Details
63-89-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
