1,2-Diaminopropane
Synonym(s):1,2-Propanediamine;Propylenediamine
- CAS NO.:78-90-0
- Empirical Formula: C3H10N2
- Molecular Weight: 74.12
- MDL number: MFCD00008089
- EINECS: 201-155-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:33
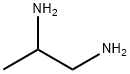
What is 1,2-Diaminopropane?
Description
1,2-Diaminopropane is a colorless liquid that is very hygroscopic and miscible with water and most organic solvents. It forms azeotropes with butanol, 2-methylpropanol, and toluene and has a strong ammoniacal odor.
Chemical properties
clear liquid
The Uses of 1,2-Diaminopropane
1,2-Diaminopropane was used as model precursor in the electron induced deposition of amorphous carbon nitride films.
The Uses of 1,2-Diaminopropane
In conjunction with cupric sulfate it is a very sensitive reagent for mercury.
Definition
ChEBI: A diamine that is propane substituted by amino groups at positions 1 and 2. Propylenediamine is commonly used as a bidentate ligand in the formation of coordination complexes.
Production Methods
Because 1,2-dichloropropane does not react smoothly with ammonia, 1,2-diaminopropane is usually prepared by aminating a mixture of 2-amino-1-propanol and 1-amino-2-propanol, which is obtained from propylene oxide and ammonia. The process differs from corresponding processes for the production of diaminoethane in that the temperature in the catalytic amination is higher (190 – 230 ℃).
What are the applications of Application
1,2-Diaminopropane is used as an intermediate for crop-protection agents, e.g., Basfungin (BASF). Analogous to the ethylenediamine derivatives, the zinc dithiocarbamate propineb acts as a fungicide. Especially in the United States, derivatives of the amine are used as fuel and lubricant additives.
General Description
A colorless liquid with an ammonia-like odor. Flash point 160°F. Density 0.87 g / cm3. Boiling point 243°F. Strongly irritates skin and tissue.
Air & Water Reactions
Very hygroscopic. Very soluble in water.
Reactivity Profile
1,2-Diaminopropane neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Flammable
Safety Profile
Moderately toxic by ingestion, sktn contact, and subcutaneous routes. A corrosive irritant to eyes, skin, and mucous membranes. Dangerous fire hazard when exposed to heat, flames, oxidizers. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. Used as an intermedate in production of petroleum and polymer additives, and surfactants. See also AMINES.
Purification Methods
Purify the diamine by azeotropic distillation with toluene. Then distil it. Store it in a CO2 free atmosphere. [Horton et al. Anal Chem 27 269 1955, Beilstein 4 IV 1255.]
Properties of 1,2-Diaminopropane
| Melting point: | -37 °C |
| Boiling point: | 120-122 °C(lit.) |
| Density | 0.87 g/mL at 25 °C(lit.) |
| vapor density | 2.6 (vs air) |
| vapor pressure | 14 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 92 °F |
| storage temp. | Flammables area |
| solubility | H2O: very soluble |
| form | clear liquid |
| pka | 9.82(at 25℃) |
| color | Colorless to Almost colorless |
| PH | 12 (100g/l, H2O, 20℃) |
| explosive limit | 2.2-11.1%(V) |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,7852 |
| BRN | 605274 |
| Dielectric constant | 10.2(Ambient) |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides. |
| CAS DataBase Reference | 78-90-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2-Propanediamine(78-90-0) |
| EPA Substance Registry System | 1,2-Diaminopropane (78-90-0) |
Safety information for 1,2-Diaminopropane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H317:Sensitisation, Skin H334:Sensitisation, respiratory |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2-Diaminopropane
| InChIKey | AOHJOMMDDJHIJH-UHFFFAOYSA-N |
1,2-Diaminopropane manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 78-90-0 98%View Details
78-90-0 98%View Details
78-90-0 -
 78-90-0 ETHYLAMINE SOLUTION 70% 99%View Details
78-90-0 ETHYLAMINE SOLUTION 70% 99%View Details
78-90-0 -
 Propylenediamine CAS 78-90-0View Details
Propylenediamine CAS 78-90-0View Details
78-90-0 -
 1,2-Diaminopropane, 98% CAS 78-90-0View Details
1,2-Diaminopropane, 98% CAS 78-90-0View Details
78-90-0 -
 1,2-Diaminopropane CAS 78-90-0View Details
1,2-Diaminopropane CAS 78-90-0View Details
78-90-0 -
 1,2-Diaminopropane CAS 78-90-0View Details
1,2-Diaminopropane CAS 78-90-0View Details
78-90-0 -
 1,2-DiaminopropaneView Details
1,2-DiaminopropaneView Details
78-90-0 -
 Propanediamine Chemical CompoundView Details
Propanediamine Chemical CompoundView Details
75178-96-0
