Malonic acid
Synonym(s):13C Labeled malonic acid;Propanedioic acid;Propanedioic acid-13C3
- CAS NO.:141-82-2
- Empirical Formula: C3H4O4
- Molecular Weight: 104.06
- MDL number: MFCD00002707
- EINECS: 205-503-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-03 09:06:10
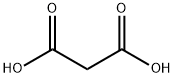
What is Malonic acid?
Description
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word (malon) meaning 'apple'.
Description
Malonic acid, formally propanedioic acid, is the second-smallest aliphatic dicarboxylic acid. (Oxalic acid?is the smallest.) It should not be confused with malic or maleic acid, both of which also contain two carboxyls.
Malonic acid is a white crystalline solid with a decomposition point of ≈135 °C. It is highly soluble in water and oxygenated solvents. It has numerous commercial uses: It is a precursor to specialty polyesters; it is used in the manufacture of barbiturates, coatings, and biodegradable containers; and it is even a component of surgical adhesives.
French chemist Victor Dessaignes reported the first synthesis of malonic acid in 1858; he made it by oxidatively decomposing four-carbon malic acid with potassium dichromate. Since then, it has been synthesized commercially starting from chloroacetic acid, diethyl malonate, and even sodium acetate.
In the past two decades, much work has been done on biobased syntheses of malonic acid. Most recently, the Berkeley, CA, venture-capital startup Lygos raised US$13 million to scale up a process that uses bioengineered yeast strains to?produce malonic acid from sugars.
Lygos, by the way, was reported by Pliny the Elder as a former name of the “town of Byzantium”. Today, of course, it’s the city of Istanbul.
Chemical properties
Malonic acid is a white crystalline solid that decomposes at approximately 135°C. It has high solubility in water and oxygenated solvents and exhibits greater acidity than acetic acid, which has a pK value of 4.75. The pKa values for the loss of its first and second protons are 2.83 and 5.69, respectively. It is slightly soluble in pyridine. It can decompose to formic acid and carbon dioxide in case of potassium permanganate. Since that malonic acid generates carbon dioxide and water after heated without pollution problems, it can be directly used as aluminum surface treatment agent.
The Uses of Malonic acid
Malonic acid is used as an intermediate in the manufacture of barbiturates and other pharmaceuticals. It is a component used as a stabilizer in many high-end cosmetic and pharmaceutical products. Malonic acid is also used as building block in chemical synthesis, specifically to introduce the molecular group -CH2-COOH. It is used for the introduction of an acetic acid moiety under mild conditions by Knoevenagel condensation and subsequent decarboxylation.
Definition
ChEBI: Malonic acid is an alpha,omega-dicarboxylic acid in which the two carboxy groups are separated by a single methylene group. It has a role as a human metabolite. It is a conjugate acid of a malonate(1-).
Preparation
Malonic acid is usually produced from chloroacetic acid.
Reaction: The chloroacetic acid is added to the reaction kettle by adding sodium carbonate aqueous solution to generate sodium chloroacetate aqueous solution, and then 30% sodium cyanide solution is slowly added dropwise, and the reaction is carried out at a predetermined temperature to generate sodium cyanoacetate. After the cyanation reaction is completed, add sodium hydroxide for heating and hydrolysis to generate sodium malonate solution, concentrate, then dropwise add sulfuric acid for acidification to generate malonic acid, filter and dry to obtain the product.
This method often does not produce a pure enough product or the pure product has an extremely low yield. Industrially, malonic acid is also produced by hydrolyzing dimethyl malonate or diethyl malonate. This manufacturing method is able to bring about a higher yield and purity, but the organic synthesis of malonic acid through these processes is extremely costly and environmentally hazardous.
What are the applications of Application
Malonic acid is acts as a building block in organic synthesis. It is also useful as a precursor for polyesters and alkyd resins, which is used in coating applications, thereby protecting against UV light, corrosion and oxidation. It acts as a cross linker in the coating industry and surgical adhesive. It finds application in the production of specialty chemicals, flavors and fragrances, polymer cross linkers and pharmaceuticals.
Reactions
In a well - known reaction, malonic acid condenses with urea to form barbituric acid. Malonic acid is also frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. The esters of malonic acid are also used as a - CH2COOH synthon in the malonic ester synthesis.
Biological Functions
Malonic acid is the classic example of a competitive inhibitor of the enzyme succinate dehydrogenase (complex II), in the respiratory electron transport chain.It binds to the active site of the enzyme without reacting, competing with the usual substrate succinate but lacking the ?CH2CH2? group required for dehydrogenation. This observation was used to deduce the structure of the active site in succinate dehydrogenase.
General Description
White crystals or crystalline powder. Sublimes in vacuum.
Air & Water Reactions
Water soluble.
Reactivity Profile
Malonic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Malonic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions Malonic acid is incompatible with strong oxidizers. Malonic acid is also incompatible with bases and reducing agents.
Hazard
Strong irritant.
Fire Hazard
Flash point data for Malonic acid are not available; however, Malonic acid is probably combustible.
Flammability and Explosibility
Not classified
Biotechnological Applications
The calcium salt of malonic acid occurs in high concentrations in beetroot. It exists in its normal state as white crystals. Malonic acid is the classic example of a competitive inhibitor: It acts against succinate dehydrogenase (complex II) in the respiratory electron transport chain.
Purification Methods
Crystallise malonic acid from *benzene/diethyl ether (1:1) containing 5% of pet ether (b 60-80o), wash with diethyl ether, then recrystallise it from H2O or acetone. Dry it under vacuum over conc H2SO4. [Beilstein 2 IV 1874.]
Properties of Malonic acid
| Melting point: | 132-135 °C (dec.) (lit.) |
| Boiling point: | 140℃(decomposition) |
| Density | 1.619 g/cm3 at 25 °C |
| vapor pressure | 0-0.2Pa at 25℃ |
| refractive index | 1.4780 |
| Flash point: | 157°C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 1 M NaOH: soluble100mg/mL, clear to slightly hazy, colorless to faintly yellow |
| form | Liquid |
| pka | 2.83(at 25℃) |
| color | White |
| PH | 3.17(1 mM solution);2.5(10 mM solution);1.94(100 mM solution) |
| Water Solubility | 1400 g/L (20 ºC) |
| Merck | 14,5710 |
| BRN | 1751370 |
| Stability: | Stable. Incompatible with oxidizing agents, reducing agents, bases. |
| CAS DataBase Reference | 141-82-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Malonic acid(141-82-2) |
| EPA Substance Registry System | Propanedioic acid (141-82-2) |
Safety information for Malonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Malonic acid
| InChIKey | OFOBLEOULBTSOW-UHFFFAOYSA-N |
Malonic acid manufacturer
JSK Chemicals
ARRAKIS INDUSTRIES LLP
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Malonic Acid CAS 141-82-2View Details
Malonic Acid CAS 141-82-2View Details
141-82-2 -
 Malonic acid CAS 141-82-2View Details
Malonic acid CAS 141-82-2View Details
141-82-2 -
 Malonic Acid pure CAS 141-82-2View Details
Malonic Acid pure CAS 141-82-2View Details
141-82-2 -
 Malonic acid CAS 141-82-2View Details
Malonic acid CAS 141-82-2View Details
141-82-2 -
 Malonic acid, GR CAS 141-82-2View Details
Malonic acid, GR CAS 141-82-2View Details
141-82-2 -
 Malonic Acid CASView Details
Malonic Acid CASView Details -
 Malonic Acid pure CAS 141-82-2View Details
Malonic Acid pure CAS 141-82-2View Details
141-82-2 -
 Malonic Acid Liquid CAS 141-82-2View Details
Malonic Acid Liquid CAS 141-82-2View Details
141-82-2
