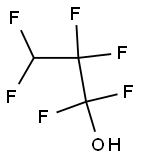
1,1,1,3,3,3-Hexafluoro-2-propanol
Synonym(s):1,1,1,3,3,3-Hexafluoro-2-propanol;1,1,1,3,3,3-Hexafluoroisopropyl alcohol, Hexafluoroisopropanol, HFP;Hexafluoroisopropanol;HFP;Sevoflurane EP Impurity C
- CAS NO.:920-66-1
- Empirical Formula: C3H2F6O
- Molecular Weight: 168.04
- MDL number: MFCD00011651
- EINECS: 213-059-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
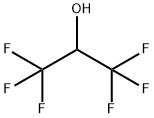
What is 1,1,1,3,3,3-Hexafluoro-2-propanol?
Description
Hexafluoro-2-propanol (erroneously called “hexafluoroisopropanol”, abbreviated HFIP) is a low-boiling solvent used in a variety of chemical applications. It first appeared in the chemical literature in a 1960 Swiss patent to Soviet Union chemists Ivan L. Knunyants and M. P. Krasuskaya, who synthesized it by reducing hexafluoroacetone1 with sodium borohydride.
After 40 years or so of being considered a laboratory curiosity, HFIP began to receive chemists’ attention. A 2017 perspective by Ignacio Colomer, Timothy J. Donohoe, and co-workers at the University of Oxford (UK) described HFIP as a highly versatile solvent. Then, in 2022, Hashim F. Motiwala, Jeffrey Aubé, and colleagues at the University of North Carolina (Chapel Hill and Wilmington) reviewed the use of HFIP in organic synthesis. They described HFIP as a polar, strongly hydrogen bond–donating solvent with the “ability to stabilize ionic species, transfer protons, and engage in a range of other intermolecular interactions”.
After describing the physical properties of HFIP and comparing it with common solvents, Motiwala, Aubé et al. discussed the advantages of using it in 20 types of organic reactions, including solvolysis, carbon–carbon bond formation, carbon–hydrogen functionalization, condensation, and cyclization. The article includes an extraordinary 629 reaction schemes in which HFIP is used as the solvent, or in some cases as a reactant.
Not to be outdone, Colomer, Donohue et al. followed up their original article by describing HFIP as “more than a polar protic solvent”. In addition to covering the same ground as Motiwala, Aubé et al., they delved into its use in electrochemical methods, organometallic and inorganic chemistry, biology, and materials and polymer science. Both author sets predict that HFIP will continue to garner many more applications in multiple scientific fields.
1. CAS Reg. No. 684-16-2.
Description
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) is aclear, colorless, oily, combustible liquid. Odor is describedasaromatic. Molecular weight = 1 68.04; Specific grav-ity = 1.596 .at 20℃; Freezing/Melting point= -4℃;Boiling point = 59℃at 760 mmHg; V apor pressure:556 mmHg at 51℃; 1468 mmHg at 75℃; Flash point: .>93℃. Hazard Identification (based on NFPA-704 MRating System): Health 3; Flammability 0; Reactivity 0.W ater soluble; solubility=≥100 mg/mL at 20℃.
Chemical properties
Colorless liquid
Chemical properties
1,1,1,3,3,3-Hexafluoro-2-propanol (HFIP) is a clear, colorless, oily, combustible liquid. Odor is described as aromatic.
The Uses of 1,1,1,3,3,3-Hexafluoro-2-propanol
1,1,1,3,3,3-Hexafluoro-2-propanol effects the native state of proteins, denaturing them as well as stabilizing the α-helical conformation of unfolded proteins and polypeptides.
The Uses of 1,1,1,3,3,3-Hexafluoro-2-propanol
Hexafluoroisopropanol is used to produce high-end chemicals, such as fluorinated surfactants, fluorinated emulsifier and fluorinated medicine, etc. HFIP is used as a solvent or cleaner in electronic industry.
The Uses of 1,1,1,3,3,3-Hexafluoro-2-propanol
It is used as a polar solvent and exhibits strong hydrogen bonding properties.It dissolves substances that are hydrogen-bond acceptors, such as amides, ethers and a wide range of polymers, including those that are not soluble in the most common organic solvents.
The Uses of 1,1,1,3,3,3-Hexafluoro-2-propanol
Usually used for preparing hexafluoroalcohol-functionalized methacrylate polymers for lithographic/nanopatterning materials.
What are the applications of Application
1,1,1,3,3,3-Hexafluoro-2-propanol is a solution-phase peptide chemistry solvent
Definition
ChEBI: An organofluorine compound formed by substitution of all the methyl protons in propan-2-ol by fluorine. It is a metabolite of inhalation anesthetic sevoflurane.
General Description
1,1,1,3,3,3-Hexafluoro-2-propanol is a solution-phase peptide chemistry solvent. This fluorinated polar solvent of high ionizing power facilitates Friedel–Crafts-type reactions, using covalent reagents in the absence of a Lewis acid catalyst. It also enhances the efficiency of rhodium(I)-catalyzed [4+2] intramolecular cycloaddition of ether-tethered alkynyl dienes and [5+2] cycloaddition of alkynyl vinylcyclopropanes. 1,1,1,3,3,3-Hexafluoro-2-propanol clusters catalyzes the epoxidation of cyclooctene and 1-octene with hydrogen peroxide.
Air & Water Reactions
Water soluble.
Reactivity Profile
1,1,1,3,3,3-Hexafluoro-2-propanol is incompatible with acids, acid chlorides and oxidizing agents.
Fire Hazard
1,1,1,3,3,3-Hexafluoro-2-propanol is probably combustible.
Potential Exposure
A specialty solvent for some poly mers; a lavatory reagent.
First aid
Eyes: Check the victim for contact lenses andremove. Flush victim's eyes with water or normal salinesolution for 20- 30 min, occasionally lifting lower andupper eyelids. Remove contaminated clothing and shoes.Get medical attention immediately. Do not put any oint-ments,oils, or medication in the victim's eyes withoutspecific instructions from a physician. Immediately trans-port the victim after flushing eyes to a hospital even ifno symptoms (such as redness or irritation) develop.Skin: Immediately flood affected skin with water andwash for at least 15 min. Remove and isolate all contami-nated clothing. Gently wash all affected skin areas thor-oughly with soap and water. If symptoms such as rednessor irritation develop, immediately calla physician.Transportthevictim toa hospital fortreatment.Inhalation: Remove victim to fresh air. If not breathing,give artificial respiration. If breathing is difficult, giveoxygen. Get medical attention immediately. Ingestion: Donot induce vomiting. If the victim is conscious and notconvulsing,give largeamountof water to dilute thechemical. Never give anything by mouth to an uncon-scious person. If symptoms (such as wheezing, coughing,shortness of breath, or burning in the mouth, throat, orchest) develop, call a physician. In all cases get medicalattention immediately. If the victim is convulsing orunconscious, do not give anything by mouth, ensure that .the victim's airway is open and lay the victim on his/herside with the head lower than the body.
Storage
Color Code- White stripe (store separately):Contact Hazard; not compatible with materials in solidwhite category. Storage precautions: you should store thischemical in a refrigerator and keep it away from ox idizingmaterials. Store away from sources of ignition. Protectiveclothing (minimum protective clothing): if Tyvek-typedisposable protective clothing is not worn during handlingof this chemical, wear disposable Tyvek-type sleeves tapedto your gloves.
Shipping
UN1760 Corrosive liquids, n.o.s., Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Distil it from 3A molecular sieves, retaining the middle fraction. It has been prepared by reduction of hexafluoroacetone in tetrahydrofuran (THF), In this case hexafluoropropanol forms a stable 1:1 complex which distils at 99-100o/760mm (n 25 1.3283), The complex is decomposed by mixing with 20% oleum and distilling in a vacuum, and the distillate is redistilled to give pure hexafluoropropan-2-ol with b 59o/760mm. The 1H NMR shows a doublet at 4.52ppm (JH,H 2Hz). The benzoyl derivative, [10315-85-2] M 272.1, has m 53.9o after crystalllisation from pentane at -50o, and its IR has at 1760cm-1. [Middleton & Lindsey J Am Chem Soc 86 4948 1964, Urry et al. J Org Chem 32 347 1967.] It has very high peptide solubilising properties, alone or with CH2Cl2 [use as a solvent: Narita et al. Bull Chem Soc Jpn 61 281 1988, Biochemistry 29 2639 1990.] It is CORROSIVE, causes severe eye irritation.
Incompatibilities
HFIP is incompatible with acids, acid chlorides, and oxidizing agents.
Waste Disposal
May be incinerated. In accor dance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be dis posed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste contain ing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 1,1,1,3,3,3-Hexafluoro-2-propanol
| Melting point: | −4 °C(lit.) |
| Boiling point: | 59 °C(lit.) |
| Density | 1.596 g/mL at 25 °C(lit.) |
| vapor pressure | 269 hPa (30 °C) |
| refractive index | n |
| Flash point: | 4,4°C |
| storage temp. | Store below +30°C. |
| solubility | miscible |
| solubility | soluble in Chloroform, Ethyl Acetate |
| pka | pK1:9.42 (25°C) |
| appearance | colorless liquid |
| form | Solid |
| color | White to yellow |
| Specific Gravity | 1.596 |
| PH | 3-4 (H2O)Aqueous solution |
| Water Solubility | 1000 g/L (25 ºC) |
| λmax | 229nm(lit.) |
| BRN | 1841007 |
| Stability: | Stable. Incompatible with strong acids, strong bases, alkali metals. |
| CAS DataBase Reference | 920-66-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanol, 1,1,1,3,3,3-hexafluoro-(920-66-1) |
| EPA Substance Registry System | 1,1,1,3,3,3-Hexafluoro-2-propanol (920-66-1) |
Safety information for 1,1,1,3,3,3-Hexafluoro-2-propanol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H361:Reproductive toxicity H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,1,1,3,3,3-Hexafluoro-2-propanol
| InChIKey | BYEAHWXPCBROCE-UHFFFAOYSA-N |
1,1,1,3,3,3-Hexafluoro-2-propanol manufacturer
JSK Chemicals
SRF LIMITED
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 1,1,1,3,3,3-Hexafluoro-2-propanol 98%View Details
1,1,1,3,3,3-Hexafluoro-2-propanol 98%View Details -
 1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
920-66-1 -
 1,1,1,3,3,3-Hexafluoro-2-Propanol (HFIP) for UV spectroscopy CAS 920-66-1View Details
1,1,1,3,3,3-Hexafluoro-2-Propanol (HFIP) for UV spectroscopy CAS 920-66-1View Details
920-66-1 -
 1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
920-66-1 -
 Hexafluoroisopropanol, 99% CAS 920-66-1View Details
Hexafluoroisopropanol, 99% CAS 920-66-1View Details
920-66-1 -
 1,1,1,3,3,3-Hexafluoropropan-2-ol CAS 920-66-1View Details
1,1,1,3,3,3-Hexafluoropropan-2-ol CAS 920-66-1View Details
920-66-1 -
 Hexafluoroisopropanol, for spectroscopy CAS 920-66-1View Details
Hexafluoroisopropanol, for spectroscopy CAS 920-66-1View Details
920-66-1 -
 1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
1,1,1,3,3,3-Hexafluoro-2-propanol CAS 920-66-1View Details
920-66-1
